PDF chapter test TRY NOW
The study of the collection of particles by using mole as the counting unit, in order to express the mass and volume of such unit particles in bulk of matter is known as mole concept.
The number of moles of a substance can be determined in several of ways depending on the data available, as follows:
- Number of moles of molecules
- Number of moles of atoms
- Number of moles of ions
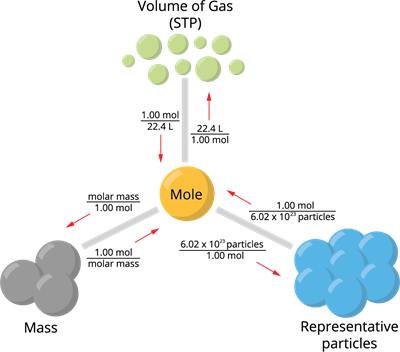
- Number of moles of a gas (standard molar volume at STP \(=22.4\) \(L\))
Where, STP stands for "Standard Temperature and Pressure" (\(273.15\) \(K\), \(1.00\) \(atm\))
Mole of atoms:
One mole of an element contains \(6.023\times10^{23}\) atoms, and it is equal to its gram atomic mass, i.e., one mole of oxygen atom contains \(6.023\times10^{23}\) atoms of oxygen, and its gram atomic mass is \(16\) \(g\).
Mole of molecules:
One mole of matter contains \(6.023\times10^{23}\) molecules, and it is equal to its gram molecular mass. i.e., one mole of oxygen molecule contains \(6.023\times10^{23}\) molecules of oxygen, and its gram molecular mass is \(32\) \(g\).
Molar volume:
One mole of any gas occupies \(22.4\) \(L\) or \(22400\) \(mL\) at STP. This volume is called molar volume.
Calculating the number of moles through different methods:
Mole concept
