
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIndustries:
Radioactive isotopes are employed as tracers to detect flaws like fractures and leaks in the manufacturing industry. Isotope like radio cobalt \(_{27}Co^{60}\) is used to detect the internal flaws in cast material.Radioactivity can be used to detect packaging flaws as well. Many companies utilise gauges with radioactive sources to monitor the levels of gases, liquids and solids.
Radio isotopes | Symbol | Uses |
Isotope of californium | \(Cf^{252}\) | In the airlines to detect the explosives in the luggage |
Isotope of americium | \(Am^{241}\) | Smoke detector in industries |
Archaeological research:
Radiocarbon dating:
Carbon found in the atmosphere exists as three isotopes like \(_{6}C^{12}\), \(_{6}C^{13}\) and \(_{6}C^{14}\). Out of which, \(_{6}C^{14}\) or \(C -14\) is an unstable isotope with a half-life period of \(5730\ years\). By the action of cosmic rays on atmospheric nitrogen, \(C -14\) is continuously produced in the atmosphere.
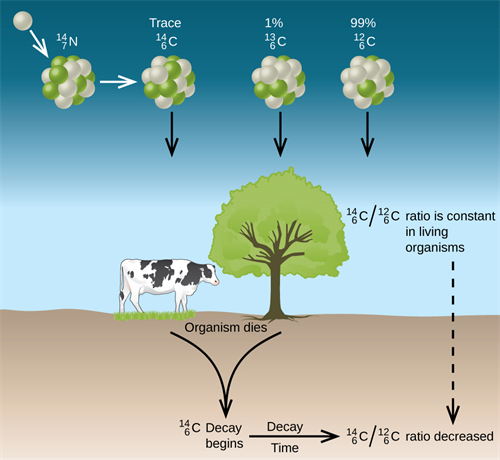
Process of carbon dating
\(C -14\) is absorbed by the cells of living things and are maintained in the constant ratio of \(C -14\) \(:\) \(C -12\). But in dead plants or animals, \(C -14\) undergoes radioactive decay without any replenishment. Hence, by determining the ratio \(\frac {(C -14)}{(C -12)}\) in fossils, their age can be determined.
The process of estimating the age by measuring the disintegration of \(C -14\) is known as carbon dating.
Radiocarbon dating is used to find the age of the Earth, fossils, historical paintings and monuments. In radiocarbon dating, the age of an object is estimated by the amount of radiocarbon present in the object. The age of earth or rocks is estimated by determining the present ratio of \(U -235\) isotope to stable lead isotope.

A fossil: Amphistium paradoxum
Important!
Our Earth is nearly \(45\ crore\ 40\ lakh\ years\) old (around \(4.54 \times 10^{9}\ years\) old).
Reference:
https://upload.wikimedia.org/wikipedia/commons/9/9b/CNX_Chem_21_03_CarbonDate.png
https://upload.wikimedia.org/wikipedia/commons/9/9c/Amphistium_paradoxum_7834.jpg