PDF chapter test TRY NOW
Important reminders:
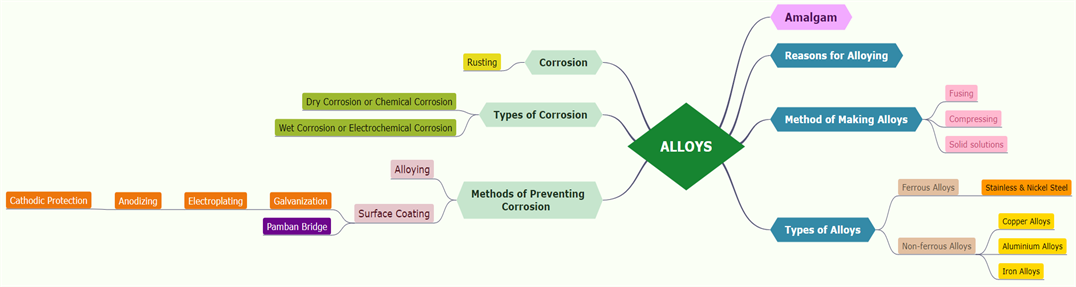
An alloy is a homogeneous mixture of two or more metals or of one or more metals with certain non-metallic elements.
An amalgam is a mixture of mercury and another metal. These alloys are formed by metallic bonding, which uses the electrostatic force of attraction between electrons and positively charged metal ions.
Reasons for alloying:
To change appearance and colour, chemical activity, to reduce the melting point, to improve hardness and tensile strength, to improve resistance to electricity.
Method of making alloys:
1. The metals are fused together.
2. Finely divided metals are compressed.
Types of alloys:
Ferrous alloys and Non-ferrous alloys based on iron component.
Ferrous alloys are stainless and nickel steel.
Non-ferrous alloys are aluminium and copper alloys.
Corrosion is a gradual destruction of metals by chemical or electrochemical reactions to the environment. It is a natural process that converts metal into its oxide, hydroxide or sulphide to lose its metallic characteristics.
Rust is chemically known as hydrated ferric oxide (it is formulated as \(Fe_2O_3\).\(xH_2O\)). Rusting results in the formation of scaling reddish-brown hydrated ferric oxide on the surface of iron and iron-containing materials.
Corrosion types:
1. Dry corrosion or Chemical corrosion
2. Wet corrosion or Electrochemical corrosion
Methods of corrosion prevention:
Alloying, surface coating, galvanization, electroplating, anodizing and cathodic protection.
Pamban Bridge is a railway bridge that connects the town of Rameshwaram on Pamban Island to mainland India. We can control the corrosion of the historical Pamban bridge by a periodical protective coating.
