
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free Demo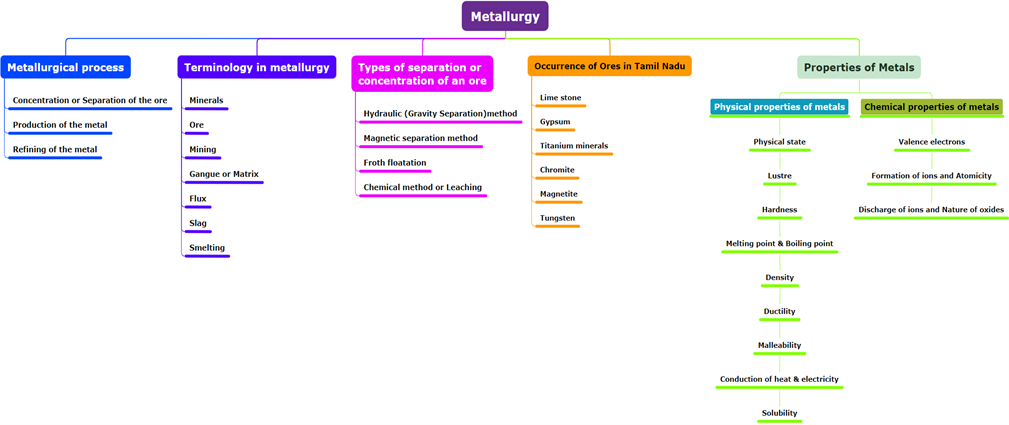
Important reminders:
A metallurgical process consists of three significant steps, which are as follows:
(i). Concentration or Separation of the ore:
It is the process of removing impurities from the ore.
(i). Concentration or Separation of the ore:
It is the process of removing impurities from the ore.
(ii). Production of the metal:
It is the process of converting ore into metal.
It is the process of converting ore into metal.
(iii). Refining of the metal:
It is the metal purification process.
It is the metal purification process.
A mineral can be a single compound or a complex mixture of different metal combinations found in the earth.
An ore is a mineral that can be readily and economically extracted on a large scale.
Mining is the process of extracting ores from the earth's crust.
Gangue or matrix refers to the rocky impurity associated with an ore.
Flux is the substance added to the ore to decrease the fusion temperature and remove the impurities.
Flux is the substance added to the ore to decrease the fusion temperature and remove the impurities.
Slag is a fusible product produced when a flux combines with a gangue during metal extraction.
Flux + Gangue → Slag
Smelting is the process of decreasing the roasted metallic oxide from the molten metal.
Types of Separation or Concentration of an Ore:
(i).Hydraulic (Gravity Separation) method.
(ii).Magnetic separation method.
(iii).Froth floatation.
(iv).Chemical process or Leaching.
(i).Hydraulic (Gravity Separation) method.
(ii).Magnetic separation method.
(iii).Froth floatation.
(iv).Chemical process or Leaching.
(i). Hydraulic (Gravity Separation) method:
Principle:
The difference in the density or specific gravity of the ore and gangue is the main principle behind this method.
Magnetic Separation Method:
Principle:
Magnetic Separation is based on the principle of magnetic properties of the components of the ore. If either the ore particles or the gangue can be attracted in a magnetic field, magnetic separation can be used.
Principle:
Magnetic Separation is based on the principle of magnetic properties of the components of the ore. If either the ore particles or the gangue can be attracted in a magnetic field, magnetic separation can be used.
(iii). Froth floatation:
Principle:
Froth floatation method is based on the principle that the metallic sulphide particles of ore are
preferentially wetted by oil (pine oil) and the gangue particles by water.
Principle:
Froth floatation method is based on the principle that the metallic sulphide particles of ore are
preferentially wetted by oil (pine oil) and the gangue particles by water.
(iv). Chemical process or Leaching:
This method is used when the ore is in a very pure form.
Occurrence of ores in Tamil Nadu:
Lime stone: Coimbatore, Cuddalore, Dindugul
Gypsum: Tiruchi and Coimbatore Districts
Titanium minerals: Kanyakumari, Tirunelveli and Tuticorin.
Chromite: Coimbatore and Salem district.
Magnetite: Dharmapuri, Erode, Salem, Thiruvannamalai.
Tungsten: Madurai and Dindugal.
Gypsum: Tiruchi and Coimbatore Districts
Titanium minerals: Kanyakumari, Tirunelveli and Tuticorin.
Chromite: Coimbatore and Salem district.
Magnetite: Dharmapuri, Erode, Salem, Thiruvannamalai.
Tungsten: Madurai and Dindugal.
Physical and chemical properties of metals.