
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free Demo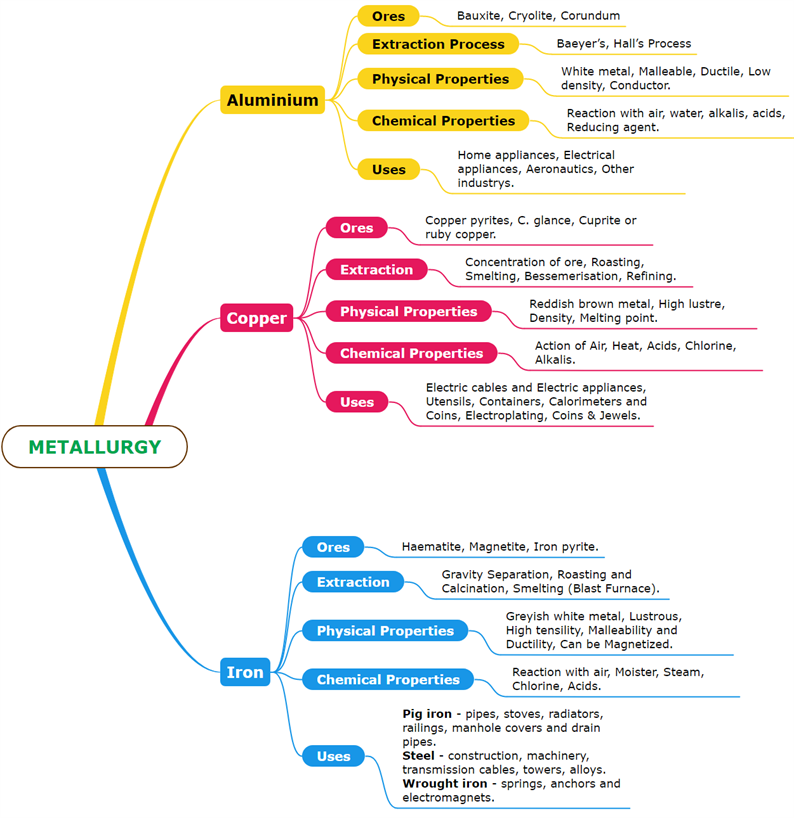
Points to remember:
The most abundant metal in the Earth's crust is Aluminium. It exists in the combined state because it is a reactive metal.
Bauxite-\(Al_2O_3.2H_2O\)
Cryolite-\(Na_3AlF_6\)
Corundum-\(Al_2O_3\)
Cryolite-\(Na_3AlF_6\)
Corundum-\(Al_2O_3\)
The chief ore of aluminium is bauxite.
Aluminium is extracted from bauxite in two steps:
Step (i). Bauxite to alumina conversion - Baeyer’s Process
Step (i). Bauxite to alumina conversion - Baeyer’s Process
Step (ii). Alumina electrolytic reduction - Hall’s Process
Physical, chemical properties and uses of Aluminium.
When a mixture of aluminium powder and iron oxide is ignited, the latter is reduced to metal. This process is known as the aluminothermic process.
The Romans called it Cuprum because they obtained it from the island of Cyprus. Copper received in the native state as well as the combined state.
Copper pyrites-\(CuFeS_2\)
Cuprite or ruby copper-\(Cu_2O\)
Copper glance-\(Cu_2S\)
Cuprite or ruby copper-\(Cu_2O\)
Copper glance-\(Cu_2S\)
The primary ore of copper is copper pyrite. It gives nearly \(76\)% of the global production of copper.
The following steps are involved in copper extraction from copper pyrites:
(i). The concentration of ore, (ii). Roasting, (iii). Smelting, (iv). Bessemerisation, (v). Refining
Physical, chemical properties and uses of Copper.
Iron is the \(2\)nd most abundant metal after aluminium. It can be found in nature as oxides, sulphides, and carbonates.
Haematite-\(Fe_2O_3\)
Magnetite-\(Fe_3O_4\)
Iron pyrite-\(FeS_2\)
Magnetite-\(Fe_3O_4\)
Iron pyrite-\(FeS_2\)
Iron chiefly extracts from Haematite (\(Fe_2O_3\)).
The following steps are involved in iron extraction from haematite:
(i). Concentration by Gravity Separation, (ii). Roasting and Calcination, (iii). Smelting (in a Blast Furnace), (a). The Lower Region (Combustion Zone), (b) The Middle Region (Fusion Zone), (c). The Upper Region (Reduction Zone).
Physical, chemical properties and uses of Iron.
Rusting: When iron is exposed to moist air, it forms a brown hydrated ferric oxide layer on its surface. This compound is known as rust, and the phenomenon of rusting is known as rusting.