
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIn the lower classes, we have learned about mixtures.
Mixture: A mixture is a substance made of two or more elements or compounds or both, physically mixed in any ratio.
The majority of the substances we encounter in our daily lives are mixtures of two or more substances. A mixture’s constituents may exist in one or more physical states.

Burning of wood
For example, when we burn wood, the smoke produced is a mixture of solid carbon and gases such as \(CO_2\), \(CO\), and others.
Do you know why lemonade tastes the same throughout the drink?
Whenever we drink lemonade juice, it tastes the same throughout. It shows that particles of sugar or salt are evenly distributed in the solution (juice).

Solutions
In some mixtures, their constituents may be separated without difficulty, whereas they cannot be separated in some cases.
Example:
Let us see the types with an example:
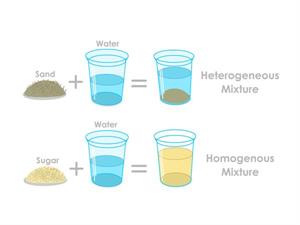
Case 1: Oil /sand and water.
Step 1: Take some water in a bowl.
Step 2: Add any compound in that water, such as oil or sand.
Step 3: Mix the water with oil or sand uniformly.
Result: You can see that the sand in the water and the oil in the water are not so mixed well. So, these kinds of mixtures with uneven compounds are known as Heterogeneous mixtures.
Difference between homogeneous and heterogeneous mixture
Case 2: Salt/ Sugar and water
Step 1: Take some water in a bowl.
Step 2: Add any compound in that water, such as sugar or salt.
Step 3: Mix the water with sugar or salt uniformly.
Result: Now, the obtained solution is the homogenous mixture. Homogenous mixtures are made by mixing any two or more compounds in the same proportions.
However, salt or sugar cannot be separated by filtration as it dissolves in water to form a homogeneous mixture. These forms of homogenous combinations are termed as “solutions”.

Filtration process
A solution is a homogeneous mixture of two or more substances that appears to be uniform in appearance.
Example:
Seawater is one of the naturally occurring solutions. We cannot imagine life on earth without the sea. Seawater is a mixture of various dissolved salts.

Seawater
Air is another example of a homogeneous mixture of gases such as nitrogen, oxygen, carbon dioxide, and so on.
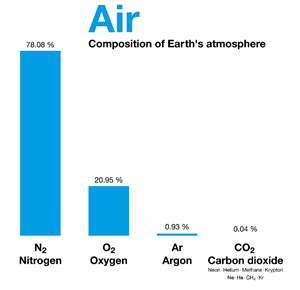
Air and its composition
All of the existing life forms on the planet are related to solutions. Plants absorb nutrient solutions from the soil in order to grow.
Absorption of minerals by plants
The majority of liquids found in the human body, such as blood, lymph, and urine, are solutions.

Cooking vegetables
Everyday human activities such as washing, cooking, cleaning, and a few others involve the formation of solutions with water.

Carbonated drinks
Similarly, the drinks we consume, such as fruit juice, aerated drinks, tea, coffee, and so on, are solutions. As a result, the ability of water to form solutions is responsible for life's sustenance.