
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoBoth forward and backward reactions occur simultaneously in a reversible reaction. When the forward reaction rate becomes equal to the rate of the backward reaction, no more product is formed. This stage of the reaction is called the 'Equilibrium state'. There can be no net change in the reaction after this stage. Therefore the reactants and products are the only ones left. As the equilibrium is attained in a chemical reaction, it is called as 'Chemical equilibrium'.
Chemical equilibrium is a state of a reversible chemical reaction in which no change in the reactants and products takes place.
Chemical equilibrium is a state of a reversible chemical reaction in which no change in the reactants and products takes place.
At equilibrium,
\(Rate of forward reaction = Rate of backward reaction\)
Explanation:
\(Rate of forward reaction = Rate of backward reaction\)
Explanation:
The rate of the forward reaction is initially greater than the rate of the backward reaction. The concentration of the reactants, on the other hand, decreases while the concentration of the products rises during the reaction. The rate of the forward reaction decreases with time, whereas the rate of the backward reaction increases, because the rate of a reaction is directly proportional to the concentration.
At some point, both rates will be equal. From this point onward, the concentrations of the reactants and products will not change with time. This is referred to as the 'Equilibrium state'.
Let us study how calcium carbonate decomposes into lime and carbon dioxide. It is a reversible process. The speed of each reaction is determined by the rate at which the reactant disappears. The reaction reaches a chemical equilibrium if it is carried out in a closed vessel.
At some point, both rates will be equal. From this point onward, the concentrations of the reactants and products will not change with time. This is referred to as the 'Equilibrium state'.
Let us study how calcium carbonate decomposes into lime and carbon dioxide. It is a reversible process. The speed of each reaction is determined by the rate at which the reactant disappears. The reaction reaches a chemical equilibrium if it is carried out in a closed vessel.
The rate of decomposition of \(CaCO_3=\)The rate of combination of \(CaO\) and \(CO_2\)
Physical as well as chemical changes can reach equilibrium. Water vapour is formed when water in a closed vessel evaporates. No water vapour escapes because the process is carried out in a closed vessel. As a result, it raises the container's vapour pressure. At some time, the water vapour condenses back into liquid water, and the process reaches equilibrium when the rate of condensation equals the rate of vapourisation.
The volume of the liquid and gaseous phases is unchanged at this point. The attained equilibrium is referred to as 'Physical equilibrium' because it represents a physical change. Physical equilibrium is a state of physical change in which all phases have the same volume.
Physical as well as chemical changes can reach equilibrium. Water vapour is formed when water in a closed vessel evaporates. No water vapour escapes because the process is carried out in a closed vessel. As a result, it raises the container's vapour pressure. At some time, the water vapour condenses back into liquid water, and the process reaches equilibrium when the rate of condensation equals the rate of vapourisation.
The volume of the liquid and gaseous phases is unchanged at this point. The attained equilibrium is referred to as 'Physical equilibrium' because it represents a physical change. Physical equilibrium is a state of physical change in which all phases have the same volume.
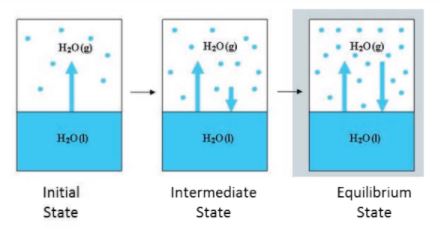
State of physical equilibrium
Characteristics of Equilibrium:
- Forward and backward reaction rates are equal in chemical equilibrium.
- The system's observable properties, such as pressure, concentration, colour, density, and viscosity, remain unaffected over time.
- The chemical equilibrium is a dynamic equilibrium since both the forward and backward reactions continue to occur even though it appears static externally. The volume of all phases is constant in physical equilibrium.
Did you know?
In a pop bottle, dissolved carbon dioxide is contained in aerated carbonated drinks (Soda). When the bottle is closed, the dissolved carbon dioxide (in the form of carbonic acid) and gaseous \(CO_2\) are in equilibrium. When you open the bottle, the gaseous form of \(CO_2\) escapes. As a result, the dissolved \(CO_2\) begins to be undissolved back to the gas phase, trying to restore the gas lost when the bottle was opened. That is why, if you keep it open for a long time, it will go 'flat'. All the \(CO_2\) will be gone, blown away in the air.