
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoPure substances are those that are completely made up of one form of particle. Physical processes cannot distinguish pure substances from other forms of matter.
Example:
Salt, sugar, oxygen, copper, iron etc.,
Based on the kind of atom, we classify pure substances as
- Elements
- Compounds
Compounds:
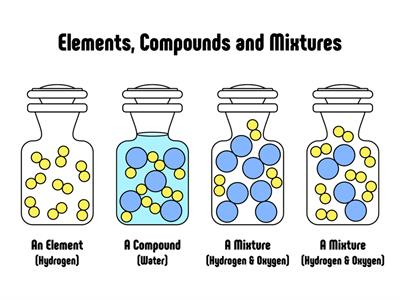
It's a form of matter created by combining two or more elements in a specific mass ratio. Chemical methods may be used to decompose it into its constituent components.
Water\(H_2O\), oxygen \(O_2\), nitrogen dioxide \(NO_2\), salt \(NaCl\), and so on.
Properties of Compounds:
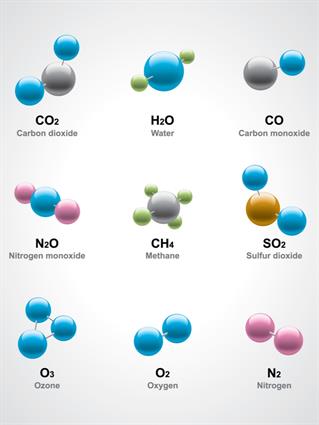
- Two or more elements are chemically mixed in a compound.
- The elements in a compound are present in a fixed mass ratio. The elements hydrogen and oxygen, for example, combine chemically in a fixed ratio to form the compound, water. This proportion will not change.
- Physical methods cannot isolate the constituents of a compound. For example, filtration, cannot isolate sodium chloride.
- The constituents of a compound lose their identities, i.e., A compound's properties vary from those of its constituent elements. Water \(H_2O\) is an example of a compound, as we chemically separate these elements; their properties differ from that of water.