PDF chapter test TRY NOW
We use various materials in our daily lives, made up of different sources, either natural or synthetic. The materials such as brush, shoe, bottles, combs, tv, plastic bags, nylon bags, mobile phones etc. are made from artificial sources. These materials can be biodegradable and non-biodegradable. The use of synthetic materials have been increased and also have become an integral part of our lifestyle.
Biodegradable materials: These sources can be decomposed by microorganisms present in the soil and recycled, for example, paper waste, food waste, animal waste etc.

Biodegradable plastics
Non-biodegradable materials: These sources cannot be decomposed by microorganisms present in the soil and are challenging to recycle, for example, plastics, nuclear waste, paints, tyres etc.

Non-biodegradable plastics
What are polymers?
The word 'polymer' is of Greek origin which denotes poly- many, mer- repeating units. Polymer is a chemical compound made up of smaller repeating units which makes it a long chain compound. These smaller repeating units are joined by covalent bonding through the process of polymerization.
Covalent bond:
The sharing of electrons takes place to achieve a stable noble gas configuration is known as a covalent bond.
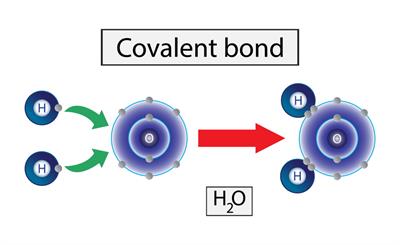
The image above shows the formation of a water molecule. Here the oxygen has six outer electrons and hydrogen as one electron. To attain the stable noble gas configuration hydrogen atom combine with the oxygen. The oxygen share two bonds with the two hydrogen atoms each and all of this atom attain a stable noble gas configuration.
Covalent bonding
Polymerization: When many monomer units combine together in a reaction, it forms a polymer.
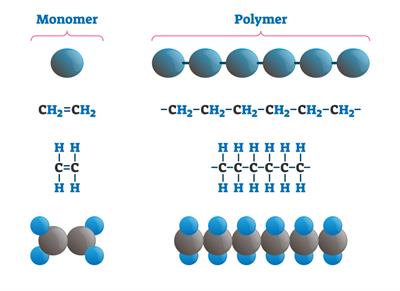
Polymerization
Let us now see how the polymerization takes place in the video below.
Let's see whether ice cubes and a polythene bag are polymers.


Polythene bag
Polythene bag: It is a polymer as it is made up of many monomer units.
Ice: The solid-state of water. Here the molecules are tightly packed, and the hydrogen and oxygen molecules are repeated throughout, which is similar to polymer. There are n number of this repeating unit, so water is a polymer.
In both the materials, there are large number molecules combined. Therefore both ice and polyethene bags are polymers.
Ice: The solid-state of water. Here the molecules are tightly packed, and the hydrogen and oxygen molecules are repeated throughout, which is similar to polymer. There are n number of this repeating unit, so water is a polymer.
In both the materials, there are large number molecules combined. Therefore both ice and polyethene bags are polymers.
