PDF chapter test TRY NOW
We have studied that in an aqueous solution, an acid can produce hydrogen ions (\(H^+\)), while a base can produce hydroxyl ions (\(OH^-\)). When an acid and a base react, a neutral product is formed, which is called salt.
According to Arrhenius theory, bases are substances that ionise in water to form hydroxyl ions (\(OH^-\)).
Some metal oxides give salt and water on reacting with acids. These are also called bases.
Bases that are highly soluble in water are called alkalis.
Example for alkalis: Sodium hydroxide, potassium hydroxide, calcium hydroxide and ammonium
hydroxide.
hydroxide.
Example for bases: Sodium carbonate, sodium bicarbonate, calcium carbonate.
When a base reacts with water, salt and water are produced.
Bases contain one or more replaceable oxide or hydroxyl ions in solution.
Important!
All alkalis are bases, but not all bases are alkalis. For example, \(NaOH\) and \(KOH\) are alkalis, whereas \(Al(OH)_3\) and \(Zn(OH)_2\) are bases.
Bases can be categorised in different ways based on the following factors:
- Acidity
- Ionisation
- Concentration
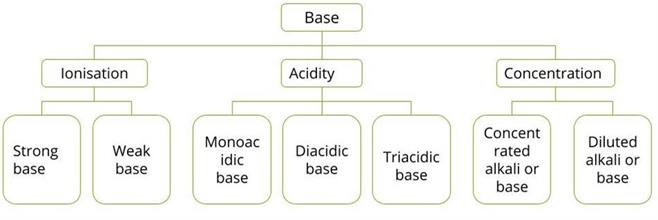 Classification of base
Classification of baseYou will learn about them in your higher classes.
