PDF chapter test TRY NOW
Oxygen is necessary for all living organisms. In \(1772\), C.W. Scheele, a Swedish chemist, first discovered oxygen. He called the gas 'fire air' or 'vital life' since it was found to support burning. In \(1774\), British scientist Joseph Priestley independently discovered oxygen. Lavoisier named this element as oxygen. The name oxygen is derived from the Greek word 'oxygenes', which means 'acid producer'. It is called so since early chemists thought that oxygen is necessary for all acids.
Occurrence:
Oxygen is the most abundant element on Earth by mass and the third most abundant element after hydrogen (\(H_2\)) and helium (\(He\)) in the universe. It can occur in both the free state and the combined state, as shown below.
Percentage occurrence:
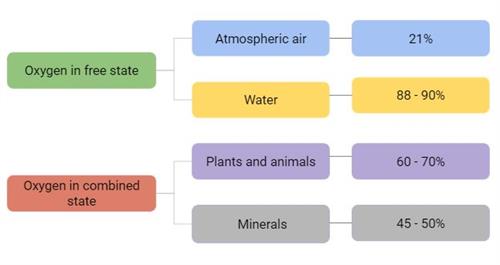
Oxygen in combined form | Occurs |
| Silicates and metal oxides | Earth's crust |
| Water | Earth's surface |
| Ozone or trioxygen molecule \(O_3\) | Upper layer of the atmosphere |
Physical properties of oxygen:
- It is a colourless, odourless and tasteless gas.
- Poor conductor of heat and electricity.
- Dissolves readily in cold water.
- At any temperature and pressure, oxygen is slightly denser than air.
- Oxygen can be converted into liquid at high temperature and low pressure. This process is called liquefaction.
- Oxygen plays a vital role in combustion.
Uses:
- It is used for cutting and welding metals as oxy-acetylene light.
- Used for removing carbon impurities from the steel.
- Living things use oxygen from the air for respiration.
- Used in the oxidising of rocket fuel.
- Scuba divers, mountaineers, astronauts and patients use artificial respiration.
- Used as explosives when mixed with powdered charcoal.
- Used for the synthesis of methanol and ammonia.
