
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoLet's see how Goldstein found proton:
In \(1886 \), Eugen Goldstein accurately predicted the existence of positively charged particles in the atom. Based on the idea that the atom is electrically neutral in nature, it needs positively charged particles to balance the negatively charged electrons.
- Goldstein redid the cathode ray experiment by using a perforated cathode.
- He passed a high voltage in a low-pressure gas.
- He noticed a faint red glow on the wall behind the cathode.
- These rays came to be known as anode rays, canal rays, or positive rays because they came from the anode.
- Anode rays were detected as a stream of positively charged particles.
Findings of Protons experiment
Anode rays Properties:
1. Anode rays follow a straight path.
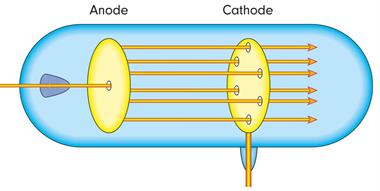
Anode rays passing
2. Anode rays are made up of material particles.
3. They are scattered by both electric and magnetic fields. Thus, they are positively charged particles.
4. The existence of cathode rays' is affected by the type of gas within the tube.
5. The particle's mass is equal to the atomic mass of the gas taken inside the discharge tube.
Protons:
- When hydrogen gas was used in a discharge tube, the positively charged particles obtained from the hydrogen gas were called protons.
- Each of these protons is created when one electron is removed from one hydrogen atom.
- An ion of hydrogen is known as a proton.