PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoMerits of Atomic Theory:
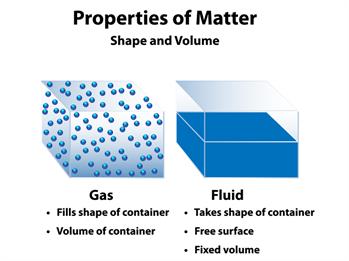
- Dalton's atomic theory explains the majority of the properties of gases and liquids.
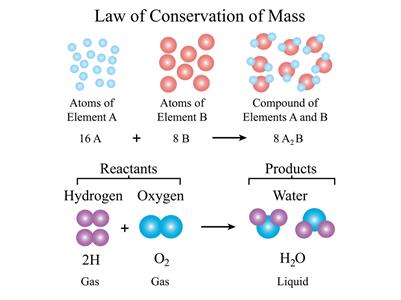
- It explains the chemical composition law and the law of conservation of mass.
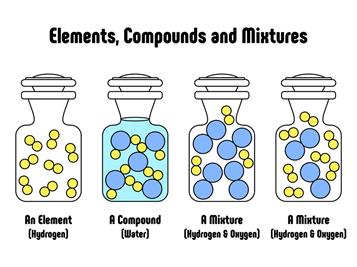
- This theory aids in the identification of molecular changes between elements and compounds.
Drawbacks of Atomic Theory:
- The atom is no longer thought to be the tiniest indivisible particle.

Divisible fruit (similarly atom also divisible into proton, electron and neutron.)
- Atoms of the same element have different masses (Isotopes).
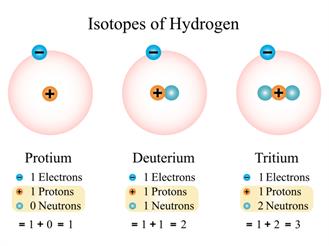
- Different elements' atoms can have the same mass. (Isobars).
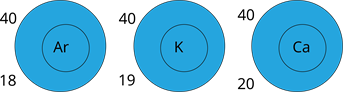
- The properties of substances made up of the same type of atoms can vary.
Example:
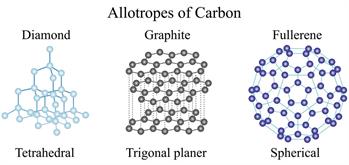
(Coal, Graphite and Diamond are consists of carbon, but they have different properties)