PDF chapter test TRY NOW
J.J. Thomson says there are two primary particles (proton and electron) in the atom.
In \(1932\), James Chadwick discovered another primary particle called 'neutron'.
But, the position of the neutron in an atom was not clear before Rutherford defined the structure of an atom.
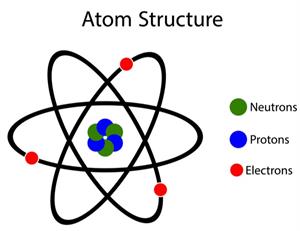
Properties of neutron:
A neutron is a non-charged neutral particle.
Mass of the neutron = Mass of proton = grams.
Basic properties of atomic particles.
Particle | Mass (grams) | Relative charge |
Electron (e) | \(-1\) | |
| Proton (p) | \(+1\) | |
| Neutron (n) | \(0\) |
Reference:
