
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoRutherford's model:
Before going to study the valency of elements, we need to know about Rutherford’s atomic model.
According to the Rutherford model, an atom consists of subatomic particles, namely, protons, electrons and neutrons.
Protons and neutrons are present in the centre of an atom, called a nucleus. Electrons are rotating around the nucleus in a circular path, called Orbits or Shells. An atom has several orbits, each with its own set of electrons. The electrons rotating in the outermost orbit are called valence electrons.
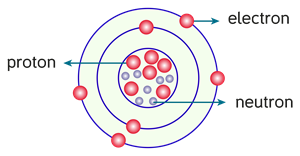
Rutherford's model
The order of electrons in the orbits is known as Electronic configuration.
Atoms of all the elements will have a stable electronic configuration, following two electrons (duplet) and eight electrons (octet) in their outermost orbit.
Example:
Helium is chemically inert since it has two electrons in the outermost orbit.
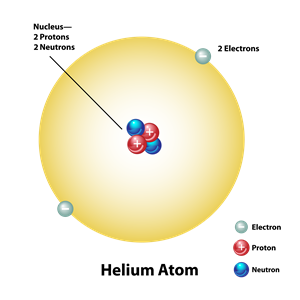
Note: Similarly, neon is also inert due to it has eight electrons in the outermost orbit.
Valency:
- The valence electrons define the chemical properties of an element in an atom, which readily participates in a chemical reaction.
- While forming molecules, since each atom has a different mixing power atoms combine in a fixed proportion. This mixing capacity of an, atom is called valency.
- Valency is the number of electrons lost, gained or shared by an atom in a chemical combination. As a result of which it becomes chemically inert.
Example:
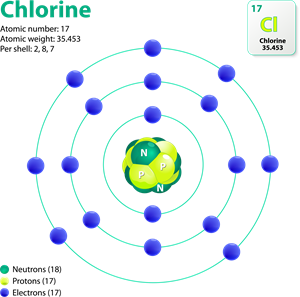
Chlorine valency
1. Chlorine atom (atomic no: \(17\)) have seven electrons in its outermost shell.
2. Getting one electron attains a stable electronic configuration, like inert gas electronic configuration.
3. Hence, chlorine has a negative valency.