PDF chapter test TRY NOW
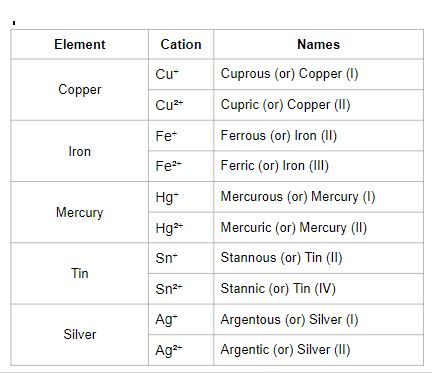
Different Valency:
- Some elements join with atoms of other elements and form more than one product; these kinds of atoms are variable valency atoms.
- Some cations have variable valencies.
Copper reacts with oxygen to forms two products, cuprous oxide (\(Cu_2O\)) and cupric oxide (CuO)
- The suffix - 'ous' is added to the end of the metal's name to indicate lower valency. For example, in Cuprous oxide, copper valency is one.
- The suffix - 'ic' is added to the end of the metal's name to indicate higher valency. For example, in Cupric oxide, copper valency is two.
Sometimes we use the Roman numeral I, II, III, IV etc., to indicate the valency of the metal.
Metals with a wide range of valencies: