PDF chapter test TRY NOW
A chemical equation is a short description of a chemical reaction using chemical symbols and formulae.
Every chemical equation contains two parts:
1. Reactants:
Participate substances in a chemical reaction
2. Products:
Formed substances in a chemical reaction
Example:
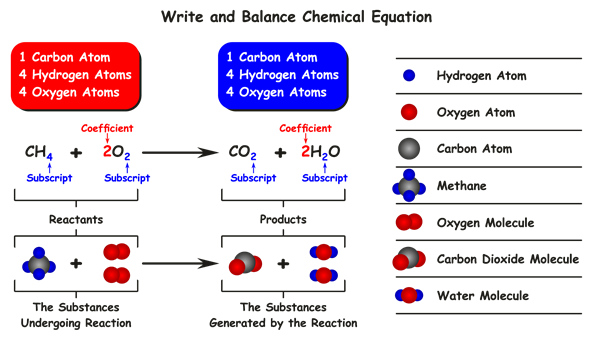
The above reactions \( CH_4 \) and \( 2O_2 \) substances participate in a chemical reaction, so these are named reactants.
The above reactions \( CO_2 \) and \( 2H_2O \) substances are formed in a chemical reaction, named products.
The steps involved in writing the skeleton equation:
A skeletal equation is written before the balanced equation of a chemical reaction is written.
Example:
The steps for writing the skeletal equation are listed below.
Step 1: Write the symbols and formulae of each reactant on the left-hand side (LHS) and connect them by plus (+) sign.
\(Mg\) + \( H_2SO_4 \)
Here Mg and \( H_2SO_4 \) are reactants connected by the plus (+) sign.
Step 2: Followed by an arrow () which is interpreted as gives or forms.
Step 3: Write the symbols and formulae for each product on the arrow's right-hand side (RHS).
Here \(MgSO_4\) + \(H_2\) are formed products.
Step 4: If the product is a gas, it should be denoted by an upward arrow (), and a precipitate should be indicated by a down arrow ().
Step 5: This written equation is called the skeleton equation (unbalanced equation).
