
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoTypes of ions:
Ions are classified into two types. They are:
- Cations
- Anions
Cations:
If an atom loses one or more electrons during a chemical reaction, it gains a positive charge. These are referred to as cations (or positive radicals).
Anions:
If an atom gains one or more electrons during a chemical reaction, it gains a negative charge. These are referred to as anions(or negative radicals).
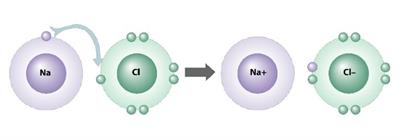
Types of ion: Anion and Cation
1. Sodium atom loses one electron to achieve stable electronic configuration, and it becomes a cation.
2. The sodium ion is described as \(Na^+\).
3. Chlorine atom gains one electron to achieve stable electronic configuration, and it becomes anions.
4. The chlorine ion is described as \(Cl^-\).
Ion Classification