PDF chapter test TRY NOW
In hydrocarbons, carbon and hydrogen atoms are linked together through different
chemical bonds. Depending on the bond between these atoms, they are classified into
chemical bonds. Depending on the bond between these atoms, they are classified into
- Alkanes
- Alkenes
- Alkynes
- Arenes
Alkanes
In alkanes, carbon-carbon atoms and carbon-hydrogen atoms are held together by single bonds. Alkanes have \(sp^3\) hybridised carbon. They have a general formula \(C_nH_{2n+2}\). They are saturated hydrocarbons.
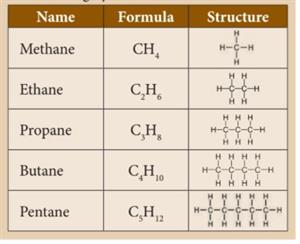
Image credits: 8th TN text book
Methane:
It is the simplest hydrocarbon, consisting of four hydrogen atoms joined to one carbon atom. It is a combustible gas that is colourless and odourless. It does not produce any hazardous substances. Methane is used as a fuel in electricity generation. Dead and decaying plants and animals release methane gas. It is a renewable energy source. Microorganisms can decompose sewage sludge to produce methane gas and impurities such as carbon dioxide and hydrogen sulphide. Methane gas can be utilised as fuel after these contaminants are removed.
Ethane:
Ethane is the second simplest alkane after methane and is colourless and odourless. It is made up of two carbon atoms and six hydrogen atoms. It is made using sodium propionate in a laboratory setting. There is only one \(C-C\) bond in it.
Propane:
It is an odourless and highly flammable gas. It is heavier than air. It is liquefied through pressurisation and commonly used as LPG (Liquefied Petroleum Gas) along with butane. Propane is used as fuel in heating, cooking and vehicles. Propane can also be used as a refrigerant.
Note: LPG cylinders are filled with propane. As it is an odourless gas, any leakage cannot be detected. Hence, mercaptan is mixed with LPG to help in the detection of any leakage of LPG.

LPG cylinders
Butane:
It is a gas at room temperature and atmospheric pressure. They are highly flammable, colourless gases that quickly vaporise at room temperature. Butane is used as fuel gas and propellant in aerosol sprays such as deodorants. Pure forms of butane can be used as refrigerants. Butane is also used as fuel for a common lighter or butane torch.
Pentane:
Pentanes are liquids with a low boiling point. They are used as fuels and solvents in the laboratory. They are also used to produce polystyrene.
Alkenes
In alkenes, carbon-carbon atoms are held together by double bonds. These are unsaturated hydrocarbons. They have a general formula \(C_nH_{2n}\).
Alkynes
In alkynes, carbon-carbon atoms are held together by triple bonds. These are also unsaturated hydrocarbons. They have a general formula \(C_nH_{2n-2}\).
These three are known as aliphatic hydrocarbons. Aliphatic hydrocarbons are straight-chain structures with no rings in their chain.
Arenes
Arenes are compounds that consist of at least one aromatic ring, hence, known as aromatic hydrocarbons.
Example: Benzene, methylbenzene, naphthalene, phenanthrene, etc.
Reference:
https://factly.in/wp-content/uploads/2016/06/new-guidelines-for-LPG-Distributorships_factly.jpg
