PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
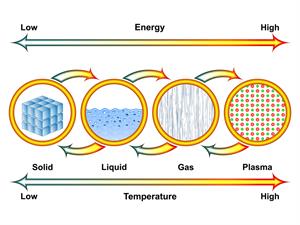
Book Free DemoAll the substances in our surroundings are made up of atoms and molecules. These atoms and molecules are always in vibratory motion. Due to this motion, substances have energy known as heat energy. This energy flows from the hot to the cold region or hot to cold substances. When any substance gets heat energy, the atoms and molecules inside the substance start to vibrate. These vibrated atoms and molecules tend to colloid with other atoms and molecules, resulting in heat energy transfer.
When we heat the object, the object's temperature will increase due to the increase in the movement of the molecules. Heat is an energy that raises the temperature of the object by inducing faster molecular movement. When the temperature of the object is different from its environment, then heat takes place.
Heat is not a matter and doesn't occupy space. Like light, sound, and electricity, it has no weight, and it is one form of energy. Heat is the kinetic energy of the particles inside the object.
Heat can be represented through the SI Unit "\(Joule\)". It is also measured as "\(Calorie\)".
Effects of Heat:
Heat energy supplied to any substance not only increases its temperature but also brings about more changes. The following are the three important effects of heat that we can see in our daily lives,
- Expansion

- Increase in temperature
- Change in state