PDF chapter test TRY NOW
When we apply the heat energy to the substance, the molecule's kinetic energy increases due to the supplied energy, increasing its temperature.
Example:
Water boiling and evaporating into water vapour (steam) when heated on the stove.
In this example, - Energy is needed to vaporise a liquid because in so doing, the molecules are separated, and attractive molecular forces are to be overcome.
The applied energy is used only to separate the molecules; no part increases their kinetic energy. There is no temperature change until a phase change is complete.
Changes of Phase:
The term change of phase means the same thing as the term change of state. The change of phase always occurs with a change of heat. If heat energy is supplied to or taken out
from a substance, it will undergo a change
from one state of matter to another. However, the temperature does not change.
During a change in state, the heat energy is used to change the bonding between the molecules.
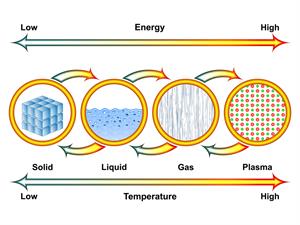
There are four states of matter in the universe - plasma, gas, liquid, and solid. But, matter on Earth exists mainly in three distinct phases - gas, liquid, and solid.
A phase is a distinctive form of a substance, and matters can change among the phases. It may take extreme temperature, pressure, or energy, but all matter can be changed.
One of the following transformations may take place,
- Solid to Liquid
- Liquid to Gas
- Solid to Gas
- Gas to Liquid
- Liquid to Solid
- Gas to Solid
Description of phase change | Term for phase change | Heat movement during phase change | Temperature change during phase change |
Solid to Liquid | Melting | Heat goes into the solid as it melts. | None |
Liquid to Gas | Vaporisation, which includes boiling and evaporation | Heat goes into the liquid as it vaporises. | None |
Solid to Gas | Sublimation | Heat goes into the solid as it sublimates. | None |
Gas to Liquid | Condensation | Heat leaves the gas as it condenses. | None |
Liquid to Solid | Freezing | Heat leaves the liquid as it freezes. | None |
Gas to Solid | Deposition | Heat leaves the gas as it freezes | None |
Note:
Water is the only matter on Earth that is found naturally in all three states - solid, liquid, and gas.
