PDF chapter test TRY NOW
A compound is formed due to the chemical combination of two or more elements in a fixed ratio by mass.
Example for compounds:
- Water (\(H_2O\))
- Backing soda (\(NaHCO_3\))
- Common salt or Table salt (\(NaCl\))
Where, water is composed of one oxygen atom and two hydrogen atoms in the ratio \(1 : 2\) by volume or \(8 : 1\) by mass.
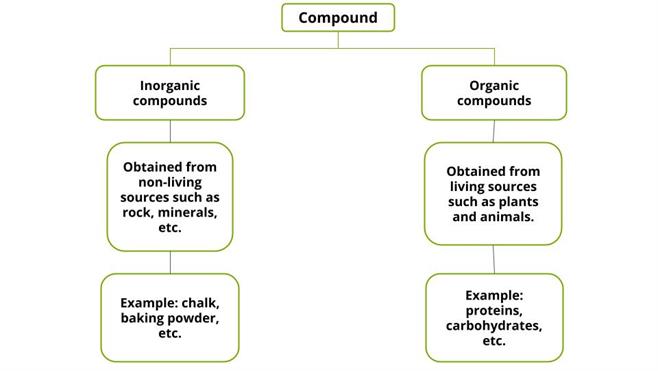
Classification of compounds:
Compounds are classified into inorganic compounds and organic compounds based on the origin of chemical constituents.
Solid compounds:
Compound | Constituent elements |
Silica (sand) | Silicon, Oxygen |
Potassium hydroxide (Caustic potash) | Potassium, Hydrogen, Oxygen |
Sodium hydroxide (Caustic soda) | Sodium, Oxygen, Hydrogen |
Copper sulphate | Copper, Sulphur, Oxygen |
Zinc carbonate (Calamine) | Zinc, Carbon, Oxygen |
Liquid compounds:
Compound | Constituent elements |
Water | Hydrogen, Oxygen |
Hydrochloric acid | Hydrogen, Chlorine |
Sulphuric acid | Hydrogen, Sulphur, Oxygen |
Acetic acid (vinegar) | Carbon, Hydrogen, Oxygen |
Gaseous compounds:
Compound | Constituent elements |
Carbon dioxide, carbon monoxide | Carbon, Oxygen |
Sulphur dioxide | Sulphur, Oxygen |
Methane | Carbon, Hydrogen |
Nitrogen dioxide | Nitrogen, Oxygen |
Ammonia | Nitrogen, Hydrogen |
Difference between the state of solids, liquids and gases:
Properties | Solid | Liquid | Gas |
| 1. Arrangement of particles | Regular arrangement | No regular arrangement | No regular arrangement |
| 2. Volume | Definite volume | Definite volume | No definite volume |
| 3. Shape | Definite shape | Non definite shape | Non definite shape |
| 4. Density | Affected by density | Affected by density | Not affected by density |
| 5. Diffusion | Minimum | Minimum | Maximum |
| 6. Force of attraction | Maximum | Maximum | Minimum |
| 7. Moment of particles | Cannot move | Slowly | Highly moveable |
| 8. Compressibility | Not compressible | Hardly compressible | Highly compressible |
Reference:
file:///C:/Users/user/Desktop/Books/8th_Science/TN/8th_Std_Term_I_Science_EM.pdf
