PDF chapter test TRY NOW
A substance that dissolves other substances (solute) is called a Solvent.
For example, consider the sugar solution. Where sugar is a solute and water is a solvent. Water is the only solvent that has the ability to dissolve more compounds than any other. It has the ability to dissolve solids like salt and sugar, liquids like honey and milk and gases like oxygen and carbon dioxide. Hence, water is also known as the universal solvent.
What is 'dissolved solids' in water?
Let us do an activity in order to understand dissolved solid.
Place a sample of tap water on a clean watch glass and place it over a beaker contains water. In the beaker, bring the water to a boil. Remove the watch glass from the burner once all of the water has evaporated and let it cool. What do you see on the display of your watch glass?
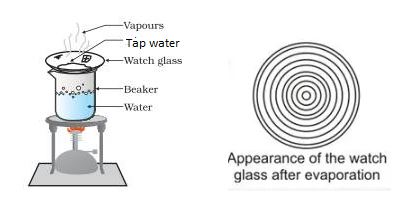
Experiment for solids dissolved in water
Image credit: Experiment
We see there are a number of concentric rings of solid matter on the watch glass. These are the dissolved solids that remain after water has evaporated. Solids dissolved in water include salts, minerals and impurities.
Dissolved solids are present in tap water, river water and well water, but not in rainfall or distilled water. Thus, after evaporation, concentric circles do not form in rainwater or distilled water.
Importance of dissolved salts in water:
- Plant's growth and development are impossible without dissolved salts in water.
- They provide taste to water.
- They provide the minerals that our body require.
- Water is used in most chemical reactions that are necessary for the survival of human body cells.
Dissolved air in water:
Air is dissolved in water, along with solids and minerals. In all-natural sources of water, the air is present as a dissolved gas. Oxygen has a higher solubility in water than nitrogen. \(35.6\)% of oxygen, nitrogen and carbon dioxide are found in the air dissolved in water.
The presence of air in water is important for the following reasons.

Air in water
- Fishes collect oxygen from the water and release water through their gills, which is necessary for living organisms to survive.
- Fish can only thrive in water if there is dissolved oxygen present.
- For photosynthesis, aquatic plants utilise dissolved carbon dioxide.
- Calcium bicarbonate is formed when carbon dioxide dissolved in water combines with limestone.
- Snails, oysters and other marine species take calcium carbonate from calcium bicarbonate to form their shells.

Fishes in water

Aquatic plants

Snails
Reference:
https://ncerthelp.com/scienceimg/09-CH2/Concept/04.jpg
https://th.bing.com/th/id/OIP.KuJhlvpGI5Vpk4AZdodhXgHaE-?pid=ImgDet&rs=1
https://th.bing.com/th/id/OIP.BScJOEXZI4aoN4oeS5QHMQHaE5?pid=ImgDet&rs=1
https://live.staticflickr.com/65535/49604016212_3745f0643c_b.jpg
https://th.bing.com/th/id/OIP.us7tCLytwb3slEyUQdtejQHaFQ?w=232&h=180&c=7&o=5&pid=1.7
