
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoRadiochemistry:
You have study in earlier chapters that elements can exist in nature as their isotopes.
Isotopes are atoms with a specific number of protons and electrons and a various number of neutrons.

Radiochemistry on DNA sequence analysis
Some isotopes are permanent and stay permanently. These are the elements that we see about us and find in nature. However, some isotopes are unstable, and they undergo disintegration by losing their energy in radiation.
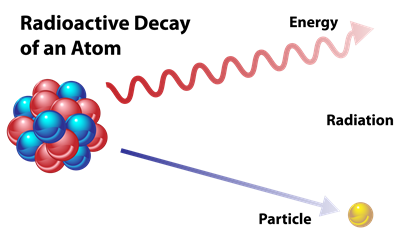
Radioactive decay:
Every element tries to achieve stability by giving, losing or gaining electrons (octet rule). Thus, the unstable isotopes of elements lose their energy in the form of radiation to become stable. This phenomenon is called Radioactive decay. The isotope which experiences radioactive decay is called Radioactive isotope or Radioisotope. This feature of isotopes is known as Radioactivity.
Radiochemistry is the learning of the chemistry of radioactive and non-radioactive isotopes. It has both natural and artificial isotopes.
Radioisotopes are used in various fields to study the nature of chemical reactions involving non-radioactive isotopes of elements.
Applications of Radiochemistry:
i. Radioisotopes can easily be identified and measured quantitatively. So they are used in radiochemistry for several applications.
ii. Radiochemistry mainly deals with the study of chemical reactions of non-radioactive isotopes using radioisotopes.
iii. In addition, it could also find applications in the medical field and environmental management. Let us list critical applications of radioisotopes.
Radiocarbon dating:
It is a process by which the age of fossil wood or animal is determined using a C-\(14\) isotope.
Diagnosis:
Radioisotope observation is helpful to diagnose and understand many diseases.
Radioisotope in Diagnosis:
Radioisotope | Diagnosis used for |
| Iodine-\(131\) | Location and detection of brain tumour, thyroid gland disorder |
| Sodium-\(24\) | Location of the blood clot and circulation disorders, pumping action of the heart |
| Iron-\(59\) | Diagnosis of anaemia, pregnancy disorder |
| Cobalt-\(60\) | Diagnosis of cancer |
| Hydrogen-\(3\) | The human body's water content |
Radiotherapy:
Many diseases are treated with radioactive isotopes. This type of treatment is named Radiotherapy.
Radioisotope in Treatment:
Radioisotope | Treatment used for |
| Gold-\(198\) | Cancer |
| Iodine-\(131\) | Hyperthyroidism and cancer |
| Phosphorous-\(32\) | Blood disorder and skin disease |
| Cobalt-\(60\) | Cancer |