
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoAs beryllium was exposed to alpha particles, James Chadwick noticed that particles with the same mass as protons were emitted. The particles that were emitted have no electrical charges. Neutron is the name given to them. \(_0n^1\) is the symbol given for neutron. Its mass is represented by superscript \(1\), and its electric charge is represented by subscript \(0\).
Properties of neutrons:
This particle was found to be electrically neutral as it was not deflected by any magnetic or electric field.
Valence electrons
We have studied how the electrons are arranged in an atom of different shells or orbits.
The electrons that are found in an atom's outermost orbit are called valance electrons.
The charge of an atom is decided by the loss or gain of electrons. An atom becomes positive when it looses an electron and negative when it gains an electron. The difference between valence and charge is that valence has no symbol, while charge has both positive and negative signs.
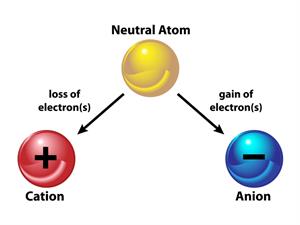
Formation of anion and cation from a neutral atom
Atoms are continually trying to achieve a stable state.
Example for an atom losing electron: Sodium
Atomic number of sodium = \(11\)
Number of electrons in \(Na\) = \(11\)
So, the electronic configuration is (\(2, 8, 1\)). It needs to lose one electron to attain a stable state.
(Loss of an electron)
Thus, the electronic configuration of \(Na^+\) is (\(2, 8\)).
Example for an atom gaining of an electron: Chlorine
Atomic number of \(Cl\) = 17
Number of electrons in \(Cl\) = 17
So, the electronic configuration of \(Cl\) is (\(2, 8, 7\)). It needs to gain one electron to attain a stable state.
Thus, the electronic configuration of \(Cl^-\) is (\(2, 8, 8\)).
What is meant by stable state?
If an atom has only one shell, the stable state is achieved when two electrons are present in it. Similarly, when an atom has two shells, the stable state is achieved when the outermost shell has eight electrons. Just a few elements such as (He\), \(Ne\) and \(Ar\) have this condition.
If an atom has only one shell, the stable state is achieved when two electrons are present in it. Similarly, when an atom has two shells, the stable state is achieved when the outermost shell has eight electrons. Just a few elements such as (He\), \(Ne\) and \(Ar\) have this condition.
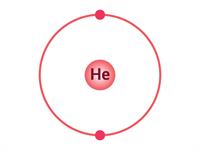
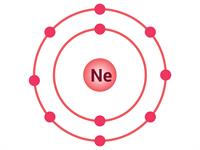
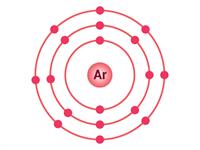
Electron distribution in \(He\), \(Ne\) and \(Ar\)
The atoms that do not have this octet are involved in bond formation by gaining, losing, or sharing electrons.
Who decides how many electrons will be involved in the formation of a bond?
The answer to this question is "Valency".