
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoFeatures of covalent compounds:
As previously stated, the nature of bonding between constituent atoms determines the properties of compounds. As a result, covalently bound chemicals differ from ionic compounds in terms of their properties.
Physical state:
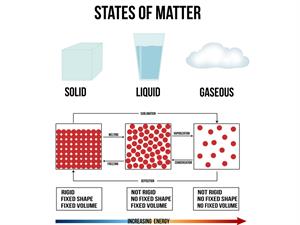
The bond can be weaker or stronger depending on the force of attraction between covalent molecules. As a result, covalent compounds exist in three states: gaseous, liquid, and solid. For example, oxygen-gas, water-liquid, and diamond-solid.
Electrical conductivity:
Covalent compounds are poor conductors of electricity because they lack charged particles (ions).

Electrical conductivity
Melting point: They have more moderate melting points than ionic compounds, except for a few covalent compounds (Diamond, Silicon carbide).

Diamond melting
Solubility:
Benzene and other non-polar solvents make covalent compounds soluble. They are insoluble in water and polar solvents.

Covalent compounds soluble

Covalent compounds are like wax
Reactions: In solutions, covalent compounds undergo slow molecular reactions.