PDF chapter test TRY NOW
Electrovalent bond:
A chemical bond formed by the electrostatic attraction of positive and negative ions.
The bond is made between two atoms when one or more electrons are transferred from the valence shell of one atom to the valence shell of the other atom.
When an atom loses electrons, it forms a cation (positive ion), and when it gains electrons, it forms an anion (negative ion).
Ionic bond:
The electrostatic force of attraction pulls these oppositely charged ions closer together, forming an ionic bond. As the bond is formed between ions, it is an ionic bond, and as the attractive forces behaving electrostatic, the bond is also called an Electrostatic bond.
Since the valence idea has been described in terms of electrons, it is also named an Electrovalent bond.
Bond formation:
Let us consider two atoms A and B. Atom A has one electron in excess, and atom B has one electron lesser than the stable octet electronic configuration. Suppose atom A transfers one electron to atom B; both the atoms will acquire a stable octet electronic configuration. As the result of this electron transfer, atom A will become a positive ion (cation), and atom B will become a negative ion (anion). These oppositely charged ions are held together by the electrostatic force of attraction called the Ionic bond or Electrovalent bond.
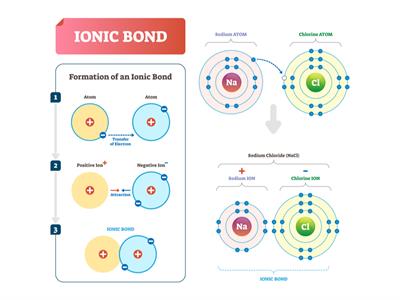
In general, an ionic bond is made between a metal and a non-metal. The compounds, including ionic bonds, are called ionic compounds. For example, Group \(1\) and \(2\) elements in the periodic table, i.e. alkali and alkaline earth metals, form ionic compounds when they react with non-metals.
