PDF chapter test TRY NOW
Why do objects sink or float on the surface of the water?
Let us discuss an activity to understand the solution for the above question.
- Take an ordinary transparent glass beaker with water.
- Place an iron nail and a piece of cork on the surface of the water.
- Observe what happens.
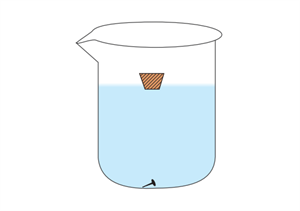
It is evident that the nail sinks. The gravitational force of the earth on the iron nail pulls it in a downward direction. At the same time, there is an upthrust of water on the nail, which pushes it in an upward direction. Since the downward force acting on the nail is greater than the upthrust of water, the nail sinks.
We already discussed the reason why the nail sinks. Now let us find out how the cork floats. This happens because of the difference in the densities.
What is density?
The density of a substance is defined as the mass per unit volume.
The unit of density is .
The density of cork is lesser than the density of water. It means that the upward force given by water on the cork is much greater than the weight of the cork.
The density of an iron nail is higher than that of the density of water. That means, the upward force given by water on the iron nail is lesser than the weight of the nail. Hence, the nail sinks. As a result objects with density less than that of the liquid floats on it.
The density of an iron nail is higher than that of the density of water. That means, the upward force given by water on the iron nail is lesser than the weight of the nail. Hence, the nail sinks. As a result objects with density less than that of the liquid floats on it.
Example:
You have a block of an unknown material, \(12\) \(cm\) long, \(11\) cm wide, and \(3.5\) \(cm\) thick. Its mass is \(1155\) grams. (a) What is the density of the material? (b) Will it float or sink in a water tank?
Solution:
The mass of the unknown material is \(1155\) grams.
Dimensions:\(12\) \(cm\) long, \(11\) cm wide, and \(3.5\) \(cm\).
(a)
(b)
Since the density of the given material is greater than the density of the water, it sinks.
