
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoHave you ever bought a mixture from a bakery?
If you noticed closely, you would have seen a variety of edible items inside that cover, likewise, the fruit salad. These are called mixtures in chemistry.

Mixture of fruits
Similar to the above example, a mixture is nothing but a combination of two or more substances.
Mixture: A mixture is a substance made of two or more elements or compounds or both, physically mixed in any ratio.
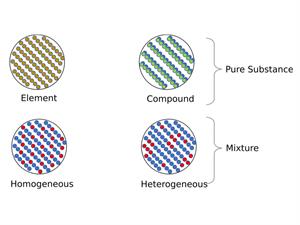
Compound and mixture
Example:
LPG: Liquefied petroleum gas
LPG is a hydrocarbon gas that is extremely flammable. It is made up of a combination of butane and propane gases. LPG is a pressurised gas that is used for heating, cooking, and motor fuel, among other things.
Air: The invisible mixture of gases that surrounds the Earth is known as air. Nitrogen, oxygen, argon, carbon dioxide, neon, helium, hydrogen, and other gases make up air.
Alloys: A metal created by combining two different types of metal.
Oil in water, sugar solution, saltwater are few more examples of mixtures.
Let us now see the difference between compounds and mixtures with the help of an activity:
Step 1: Combine powdered iron filings and sulphur in a mixing bowl.
Step 2: Make two equal portions using the mixture.
Step 3: The first half of the mixture should be kept separately, while the second half should be heated.
\(Iron + sulphur\overset{Heating}{\rightarrow} Iron sulphide\)
Observation: When heated, a black brittle substance (iron sulphide) forms.
Result: The iron sulphide (compound) that forms have completely different characteristics than the iron and sulphur combination.

\(Iron sulphide\) \(=\) \(compound\)
Here when the magnetic field is applied, it has no effect.

Iron and sulphur mixture on exposure to magnetic field
\(Iron + sulphur\) \(=\) \(mixture\)
Here when the magnetic field is applied, the iron powder gets attracted.

Magnetic effect on mixture
Thus, from the above activity, we proved that the properties of the elements of a mixture are displayed. In contrast, the properties of the individual elements are not visible in compounds.
Now let us also see few more differences between a compound and a mixture:
Mixture | Compound |
| There are two or more substances in a mixture. | A single substance is referred to as a compound. |
| The component can be present in any amount. | The elements are present in predetermined amounts. |
| Physical procedures can easily separate the components. | Only one or more chemical processes will separate the ingredients. |
In this section, we have learned the distinction between a mixture and a compound. In the following section, we will look at the types of mixtures.