
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIntroduction of elements:
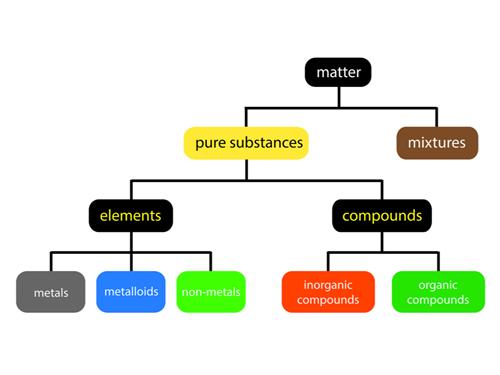
Everything in this universe is made of matter. We live in a world of substances with great diversity. The combination of various elements produces substances. All the elements are unique in their nature and property. Over the years, scientist have looked for different ways to categorize these elements according to their properties.
By \(1800\), the scientist knew only \(31\) elements. In \(1865\), their number had increased to \(63\). There are now \(118\) elements known. In the process, scientists have collected more and more information about the properties of various elements as they were discovered. They began looking for some pattern in their properties to study such elements with ease.
Let us discuss the concepts of classification of elements proposed by various scientists from the early to the modern period.
Periodic table:
The chemical elements are arranged in increasing order of atomic number.
Classification of elements:
Dobereiner’s triads:

In \(1817\), Johann Wolfgang Dobereiner, a German chemist, proposed grouping elements based on their relative atomic masses. He organised three elements into each group. He referred to these groups as 'triads' (tri-three).
When the \(3\) elements in a triad are arranged in ascending order of their atomic masses, Dobereiner discovered that the atomic mass of the middle element is nearly equal to the average of the atomic masses of the other two elements. This is known as Dobereiner's law.
Dobereiner's law of triads:
Example:
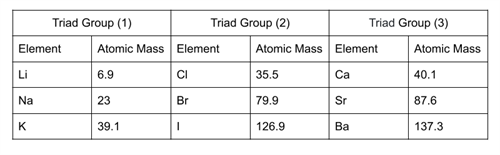
In the triad group \(1\), the arithmetic mean of atomic masses of \(1\)st and \(3\)rd elements, (\(6.9\) + \(39.1\))/\(2\) = \(23\). So, the atomic mass of \(Na\) (middle element) is \(23\).
In the triad group \(2\), the arithmetic mean of atomic masses of \(1\)st and \(3\)rd elements, (\(35.5\) + \(126.9\))/\(2\) = \(79.9\). So, the atomic mass of \(Br\) (middle element) is \(79.9\).
Drawbacks of Dobereiner's law:
- Dobereiner could recognise only three triads from the elements known at that time, and all elements could not be listed in the form of triads.
- The law did not apply to elements with low and high atomic masses.