
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
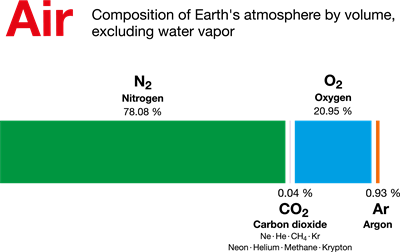
Book Free DemoWe know that our air is a mixture of various gases. Let's get to know in what composition each gas is present in the air. Nitrogen and Oxygen constitute 99% of the total volume of air. The remaining is 1% is constituted by gases such as Argon (0.93%), Carbon-di-oxide, (0.03%), Neon (0.0018%), Helium(0.0005%), Ozone (0.00006%) and Hydrogen (0.00005%). Krypton, Xenon and Methane are also present in the trace.
The Water vapour (0 - 0.4%) present in the atmosphere plays a significant role in predicting weather phenomenon.
The Water vapour (0 - 0.4%) present in the atmosphere plays a significant role in predicting weather phenomenon.
When the altitude increases, the atmospheric pressure decreases, i.e. density of the air decreases with an increase in altitude. Despite the change in density of the atmosphere with altitude, the composition stays the same, with one exception i.e. ozone layer. In the ozone layer, there is a greater concentration of ozone molecules compared to any other layer in the atmosphere.
You could have seen dust particles floating in the air when sun rays enter a building through a small opening. From this, we might have observed that the atmosphere, in addition to gases, also contains solid particles such as dust particles, salt particles, pollen grains, smoke, soot, volcanic ashes etc. These solid particles are important for condensation and precipitation.
Importance of Atmospheric Gases
- Oxygen: Most important for the survival of living organisms (breathing).
- Carbon dioxide absorbs heat and keeps the atmosphere warm by insulation and radiation.
- Nitrogen: Acts as a diluent and is chemically inactive.
- Ozone: Protects the earth from harmful ultraviolet radiation.
- The solid particles in the atmosphere act as nuclei on which water vapour condense to form precipitation.