PDF chapter test TRY NOW
Decomposition reactions are the opposite of combination reactions.
A single reactant breaks down into two or more products under suitable conditions (current, heat and light).
\(AB\) → \(A\) + \(B\)
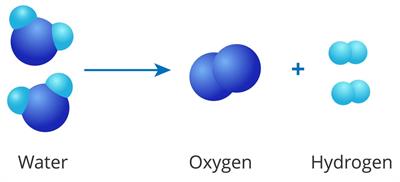
Water decomposition reaction
Activity \(1.5\): Decomposition of ferrous sulphate.
Materials required:
-Ferrous sulphate crystals
-One boiling test tube
-A burner and
-A tong
Experimental procedure:
Step 1: Take \(2\) g of ferrous sulphate crystals in a boiling test tube.
Step 2: Heat the test tube over a burner while holding it with a tong.
Step 3: Observe what happens when the crystals are being heating inside the test tube.
Observation:
- We observed that the colour of the crystals changed from green to brown (because heating the crystal lost all its water content).
- The smell of gas comes from the test tube while the crystal is burning.

Decomposition of ferrous sulphate
Result:
The brown colour powder formed after burning the crystal is ferric oxide.
The smell of the gas coming from the test tube while burning the crystals is sulphur dioxide.
Decomposition reaction:
\(2FeSO_4\)(s) \(Fe_2O_3\)(s) + \(SO_2\)(g) + \(SO_3\)(g)
(Ferrous sulphate) (Ferric oxide)
Conclusion:
In this reaction, you can notice that a single reactant breaks down to give simpler products, known as a decomposition reaction. Here Ferrous sulphate crystals (\(FeSO_4.7H_2O\)) lose water when heated, and the colour (green to brown) of the crystals changes. It then decomposes to ferric oxide (\(Fe_2O_3\)), sulphur dioxide (\(SO_2\)) and sulphur trioxide (\(SO_3\)). Ferric oxide is a solid, while \(SO_2\) and \(SO_3\) are gases.
Thermal decomposition:
When a single substance is heated, it decomposes into two or more simple components, known as thermal decomposition. Because heat is required to break the bonds in the substance, the reaction is usually endothermic.
Example: Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated.
Let us do one activity for thermal decomposition reaction that is ammonium chloride decomposition in an open container.
Materials required:
-Ammonium chloride
-Nessler’s reagent \(K_2[HgI_4]\)
-Blue litmus paper
-Stand with clamp
-Tripod stand
-Burner
-China dish
-Wire gauge and
-Funnel
Experimental procedure:
Step 1: Take \(5\) g of ammonium chloride in a dry and clean china dish.
Step 2: Place the china dish on a wire gauge and use a tripod stand to support it.
Step 3: Invert a clean and dry funnel over the china dish with the sample.
Step 4: Heat the ammonium chloride sample in the china dish.
Step 5: Vapours that are produced come out from the stem of the funnel. (See if the china dish produces any liquid).
Step 6: Bring a strip of filter paper dipped in Nessler's reagent to the tip of the funnel. Observe how the colour of the filter paper changes.
Step 7: Bring a piece of wet blue litmus paper to the funnel's tip. Observe how its colour changes.
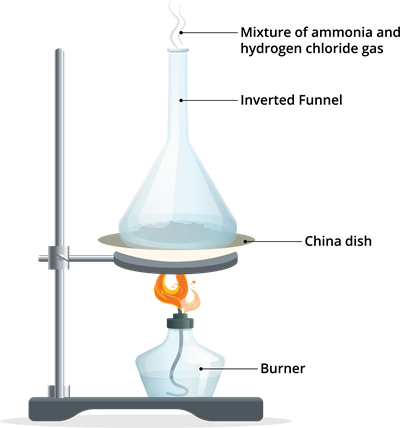
Ammonium chloride decomposition
Observation:
No. | Experiment | Observations | Inference |
1 | Nessler’s reagent test | Brown precipitate | Presence of ammonia |
2 | Litmus paper test | Red litmus paper turns into blue | Presence of ammonia |
Based on Nessler's reagent and the litmus paper test results, infer the presence of ammonia and hydrogen chloride gases in the vapours coming from the funnel. When heated in an open system, assume that ammonium chloride decomposes into ammonia and hydrogen chloride gases.
\(NH_4Cl\)(s) \(NH_3\)(g) + \(HCl\)(g)
Conclusion:
In this experiment, you can notice that a single reactant breaks down to give simpler products, known as a decomposition reaction. Here ammonium chloride undergoes a decomposition reaction to produce ammonia and hydrogen chloride gases.
In this reaction, solid ammonium chloride is directly converted into a gaseous state without changing to liquid. It is thus a sublimation reaction.
Example:
Decomposition of calcium carbonate to produce calcium oxide and carbon dioxide on heating is an essential decomposition reaction used in different industries. Calcium oxide is called lime or quick lime. It has many uses - one such use is the production of cement. When a decomposition reaction is carried out by heating, it is termed as thermal decomposition.
\(CaCO_3\)(s) \(CaO\)(s) + \(CO_2\)(g)
(Limestone) (Quick lime)
