
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoLet us do an activity to understand the process of decomposition.
Activity \(1.6\): Decomposition of lead nitrate.
Materials required:
-Lead nitrate powder \(2\)
-Boiling tube
-Tongs and
-Burner
Experimental procedure:
Step 1: Take \(2\) g white lead nitrate powder in a boiling tube.
Step 2: Heat the boiling tube over the flame of the burner using the tong.
Step 3: Observe what happens when you are heating lead nitrate in the boiling tube.
Observation:
- Emission of brown fumes (brown fumes coming out of the test tube).
- The white lead nitrate, after heating, converted into a brown powder.
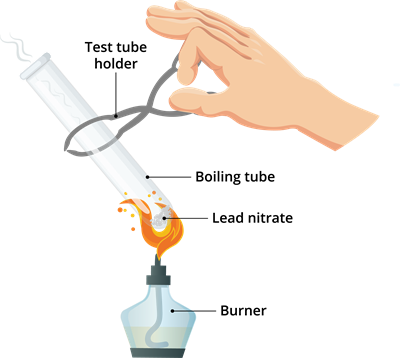
Decomposition of lead nitrate
Result:
The brown fumes coming out of the boiling tube are nitrogen dioxide.
The brown powder formed after heating the white lead nitrate is lead oxide.
Decomposition reaction:
\(2Pb(NO_3)_2\)(s) \(2PbO\)(s) + \(4NO_2\)(g) + \(O_2\)(g)
(Lead nitrate) (Lead oxide) + (Nitrogen dioxide) + (Oxygen)
Conclusion:
In this reaction, you can notice that a single reactant breaks down to give simpler products. This is known as a decomposition reaction. Here lead nitrate decomposes into lead oxide, nitrogen dioxide, and oxygen.