
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoA displacement or replacement reaction occurs when a part of one reactant is displaced or replaced by another reactant.
\(AB + CD → AD + CB\)
AC are positive ions (cations)
BD are negative ios (anions)
Let us perform an activity to see the process of displacement reaction.
Example:
Activity \(1.9\): To investigate the chemical reaction of an iron nail with an aqueous copper sulphate solution.
Materials required:
-Three iron nails
-Two test tubes
-Measuring cylinder (\(50\) mL)
-Copper sulphate
-Water
-Dilute sulphuric acid
-Sandpaper
-Thread
-Stand with clamp
-Test tube stand and
-Single bored cork
Experimental procedure:
Step 1: Take two iron nails and clean them by rubbing them with sandpaper.
Step 2: Take \(20\) mL of distilled water in a clean test tube and dissolve \(1.0\) g of copper sulphate in it. Add \(2\) or \(3\) drops of dilute sulphuric acid to it to check the hydrolysis of \(CuSO_4\) in water. Label this test tube as A.
Step 3: Transfer \(10\) mL of the copper sulphate solution from test tube A to a clean test tube. This test tube will be labelled as B.
Step 4: Tie one iron nail with a thread and dip it carefully in copper sulphate solution in test tube B through a boring cork [as shown in the below figure] for about \(15\) minutes. Keep the other iron nail separately for comparison.
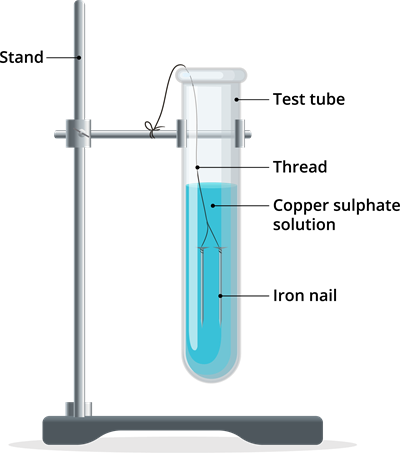
Iron copper displacement reaction
Step 5: Take the iron nail from the copper sulphate solution after \(15\) minutes.
Step 6: Compare the intensity of the blue colour of copper sulphate solution in tubes A and B before and after the experiment, as well as the colour of an iron nail dipped in copper sulphate solution versus one kept separately. Record your observations.
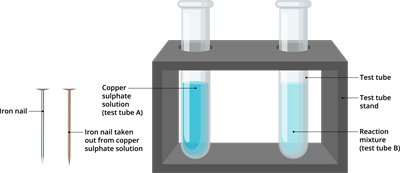
Result of iron copper displacement reaction
Observation:
- The colour of the iron nails immersed in the copper sulphate solution in test tube B became brownish.
- In test tube B, the colour of the copper sulphate solution has faded.
- Before the experiment, the colour of the copper sulphate solution was blue, after the experiment, it turns to light green.
- Similarly, the colour of the iron nail before the experiment, was grey, after the experiment it turns into red-brown.
Result:
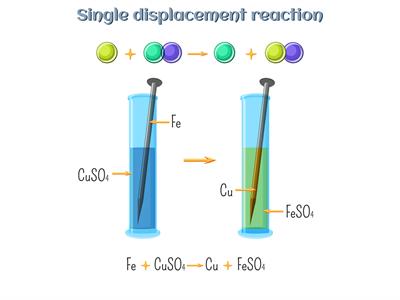
We know that a highly active iron (Fe) element displaces a less active copper (Cu) element in a displacement reaction. So, iron displaces copper from its salt solution copper sulphate because of the displacement reaction.
Displacement reaction:
\(Fe\)(s) + \(CuSO_4\)(aq) → \(FeSO_4\)(aq) + \(Cu\)(s)
(Copper sulphate) → (Iron sulphate)
Conclusion:
In this activity, iron has displaced or replaced another element, copper, from the copper sulphate solution. This reaction is called the displacement reaction. Here, copper gets displaced from copper sulphate solution, which you can see on the iron nails. The brownish colour is deposited on the iron nails. Iron took the place of copper in copper sulphate solution in test tube B. The faded solution is iron sulphate.
Example:
Some other examples of displacement reactions are-
\(Zn\)(s) + \(CuSO_4\)(aq) → \(ZnSO_4\)(aq) + \(Cu\)(s)
(Copper sulphate) → (Zinc sulphate)
(Copper sulphate) → (Zinc sulphate)
\(Pb\)(s) + \(CuCl_2\)(aq) → \(PbCl_2\)(aq) + \(Cu\)(s)
(Copper chloride) → (Lead chloride)
Zinc and lead both are more reactive than copper. They replace or displace copper from its compounds.