PDF chapter test TRY NOW
A reaction in which an exchange of ions between the reactants takes place is called a double displacement reaction. (Or)
In a double displacement reaction, the cations and anions of two compounds change positions.
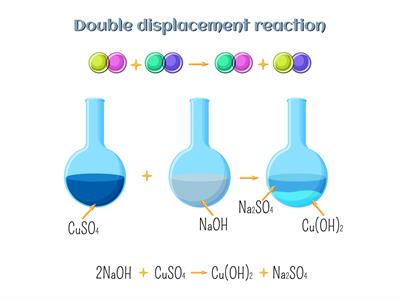
(\(A^+B^-\)) + (\(C^+D^-\)) → (\(A^+D^-\)) + (\(C^+B^-\))
A and C are cations (positive ions)
B and D are anions (negative ions)
Let us perform an activity to see the process of double displacement reaction.
Example:
Activity \(1.10\): Double displacement reaction between sodium sulphate - barium chloride
Materials required:
-Sodium sulphate solution
-Barium chloride solution
-Two test tubes and
-Measuring cylinder (\(50\) mL)
Experimental procedure:
Step 1: Take \(3\) ml of sodium sulphate solution in a test tube and label it as A.
Step 2: Take \(3\) ml of barium chloride solution in another test tube and label it as B.
Step 3: Pour the solution from test tube A into test tube B.
Step 3: Pour the solution from test tube A into test tube B.
Step 4: Mix the solutions with light shaking.
Step 5: Observe the changes in the colour of the solutions as described in the below observation.
Observation:
- Before mixing, the solutions in test tubes A and B are colourless.
- Just after we mixed the solutions, we saw the formation of white precipitates. This white substance was insoluble in water.

Double displacement reaction
Result:
The white substance (precipitate) formed, which is insoluble in water, is barium sulphate. Any reaction that forms a precipitate can be called as a precipitation reaction.
The transparent solution which you observe around the white substance is sodium chloride.
Double displacement reaction:
\(Na_2SO_4\)(aq) + \(BaCl_2\)(aq) → \(BaSO_4\)(s) + \(2NaCl\)(aq)
(Sodium (Barium → (Barium (Sodium
sulphate) chloride) sulphate) chloride)
sulphate) chloride) sulphate) chloride)
Conformation test of barium:
\(BaSO_4\)(s) + \(HCl\)(aq) → \(BaCl_2\)(aq) + \(SO_2\)(g)
When barium sulphate reacts with dilute hydrochloric acid, it forms water-soluble colourless product barium chloride. This colourless product formation confirms the presence of barium in barium sulphate. The white precipitate of barium sulphate is disappeared.
Under the favourable condition, it suggests that the substances which produce ions \(SO_4{^2}{^-}\) and \(Ba^2{^+}\) in water result in a precipitation reaction.
Conclusion:
In this activity, the reactants exchanged their ions to make two new products because of the double displacement reaction. The white precipitate of \(BaSO_4\) is produced by the reaction of \(Ba^2{^+}\) and \(SO_4{^2}{^-}\). The sodium chloride that is produced as a byproduct remains in the solution. The reactions in which ions are exchanged between the reactants are known as double displacement reaction.
Example:
After mixing, lead nitrate reacts with potassium iodide to produce insoluble yellow lead iodide.
(i). What is the colour of the precipitate that produced? Can you name the precipitated compound?
The colour of the precipitate produced is yellow, and the name of the precipitate is lead iodide.
(ii). Write a balanced chemical equation for this reaction.
(\(PbNO_3)_2\) + \(2KI\) → \(PbI_2\) + \(2KNO_3\)
(iii). Is this also a double displacement reaction?
Yes, this is an example of a double displacement reaction.
