
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIon:
An ion is formed when an atom is either receives or loses electrons. The electrical charge of ions is either positive or negative.
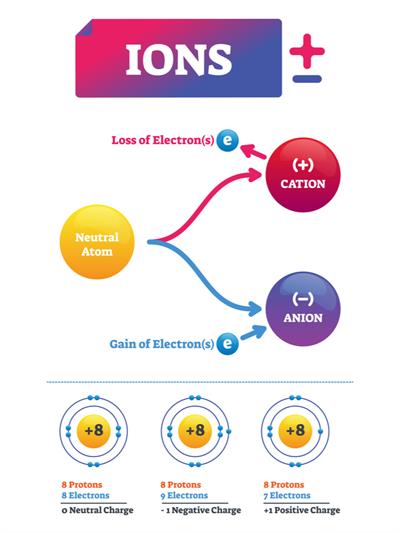
Cation:
When an atom loses an electron, what is the charge of the ion?
An atom with the same number of protons and electrons has no overall charge. However, if a negatively charged electron is removed, the atom will have more protons and thus a positive charge. Cation refers to ions that have a positive charge.
Anion:
When an atom gains an electron, what is the charge of the ion?
The atom will have more electrons and a negative charge if it gains an electron. Negatively charged ions are known as anions.
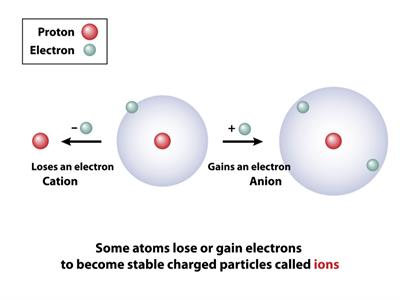
Ionization:
If a neutral atom gains or loses any one of its electrons, then it becomes an ion. This process is known as ionization.
Example:
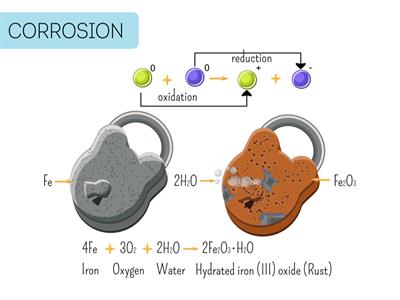
i). Ionization is a naturally occurring reaction. The rusting of iron is a good example of ionization. The rusting reaction occurs when oxygen in the form of water or moisture will reacts with the iron. It is then, the iron will become iron oxide by giving two-electron to oxygen.
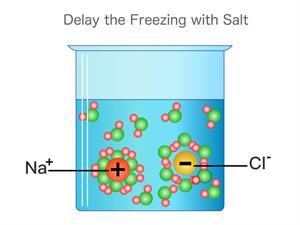
ii). Consider a compound Sodium chloride \(NaCl\). This compound's constituent particles are positively charged sodium ions \(Na^+\) and negatively charged chloride ions \(Cl^–\).
When an atom's outermost electron energy level completes, it is stable. An atom will give, take, or share electrons to fill its outermost shell.
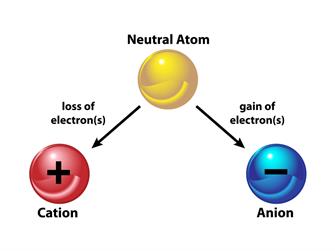
Note: Gains of electron = Anion (negative charge)
Loss of electron = Cation (positive charge).