
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoA molecule is the smallest particle of an element or compound capable of an independent life and exhibiting all of the substance's properties.
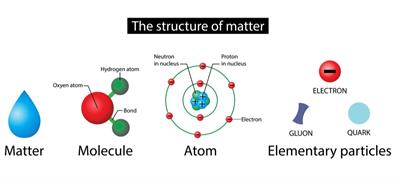
Molecule:
In general, a molecule is a set of two or more atoms that are chemically bound or held together by attractive forces.
Atoms from the same or different elements may combine to form a molecule.
Atoms from the same or different elements may combine to form a molecule.
Example:
For simple understanding, consider a water molecule; two elements, hydrogen and oxygen atoms combined to form a water molecule.
Calcium oxide \(CaO\) contains two elements, calcium and oxygen atoms combined to form a calcium oxide molecule.
Glucose \(C_6H_{12}O_6\) contains three elements, carbon, hydrogen and oxygen atoms combined to form a glucose molecule.
Sodium chloride \(NaCl\) contains two elements; hydrogen and oxygen atoms combined to form a sodium chloride molecule.
Chlorine \(Cl_2\) contains two elements of a chlorine atom.
Oxygen \(O_2\) contains two elements of the oxygen atom.
Atomicity:
We can distinguish the molecules respective to the number of elements they combined to form a molecule. We call this atomicity .i.e. the number of atoms constituting a molecule.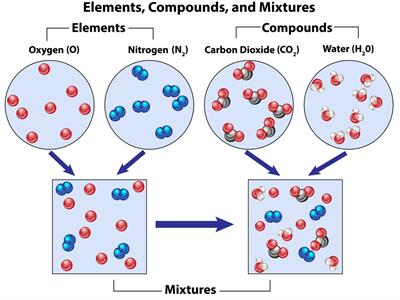
The number of atoms present in a single molecule is termed as its atomicity.

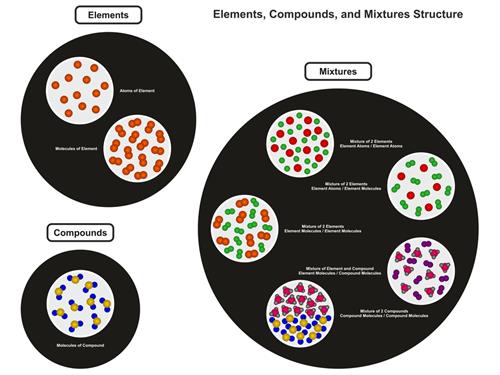
Molecules of Elements:
The atoms that make up an element's molecules are all of the same nature.
- Monoatomic Elements: In their molecular form, certain elements are monatomic, meaning they are made up of just one atom (mono-atomic). Helium \(He\) is an example of a monoatomic element.
- Diatomic Elements: If the molecule constitutes two atoms, we can classify it as a diatomic molecule. In their molecular shape, other elements have two or more atoms. For example, each molecule of hydrogen \(H_2\), oxygen \(O_2\), and chlorine \(Cl_2\), has two atoms.
- Triatomic Elements: If another atom is combined with , it forms ozone, which is a triatomic molecule.
Note:
Monoatomic and diatomic molecules are more stable than triatomic molecules.
Most non-metal elements are diatomic.
Classification of elements | Name of the elements | Symbol | Atomicity |
Metals | Copper | \(Cu\) | Monoatomic |
Aluminum | \(Al\) | Monoatomic | |
Sodium | \(Na\) | Monoatomic | |
Iron | \(Fe\) | Monoatomic | |
Non-metals | Nitrogen | Diatomic | |
Hydrogen | Diatomic | ||
Oxygen | Diatomic | ||
Helium | \(He\) | Monoatomic | |
Chlorine | Diatomic | ||
Ozone | Triatomic | ||
Phosphorous | Tetra-atomic |
Molecules of Compounds:
Compound molecules contain atoms from two or more separate elements.
Example:
Water (H2O), has three atoms: two hydrogens \(H\) atoms and one oxygen \(O\).
Methane (CH4), has with five atoms: one carbon \(C\) and four hydrogen \(H\) atoms.