
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoChemistry deals with matter and its changes, where substances change entirely into a different one. These chemical changes are governed by specific empirical laws known as the laws of chemical combinations.
Antoine Lavoisier
The laws of chemical combination elaborate the fundamental principles obeyed by interacting atoms and molecules. Antoine Lavoisier and Joseph L. Proust proposed these laws by conducting many experiments.
The laws of chemical combinations are
- Law of conservation of mass
- Law of constant proportions or law of definite proportions
Law of Conservation of Mass:
When we are burning woods, we can observe its entire body is transforming to ashes, soot, and gases.
But what if I say the total mass of the wood before burning and the total mass of the ashes, soot, and gases are equal? Would you believe it?

Scientist observed the chemical experiments and stated that the mass of the reactants at the beginning of a reaction would be equal to the mass of the products at the end of the reaction.
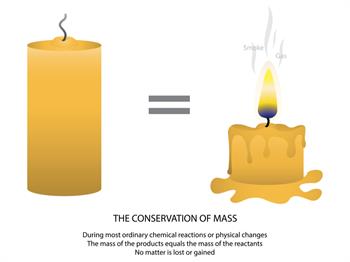
Note: "Some quantity of mass is converted as heat and light energy. It can't be calculated with mass."
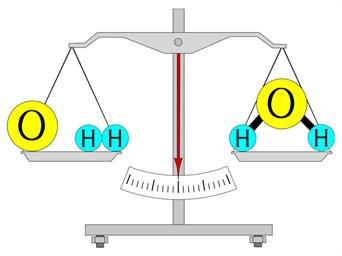
“The mass in an isolated system can neither be created nor be destroyed but can be transformed from one form to another”.
i). The simple example of this law can be shown with the help of the melting process.
\(100\) grams of ice cubes in a glass will become liquid when allowed to melt. The mass of both the starting and ending substance will be equal, according to this law.

ii). Sugar solution preparation: If we mix sugar with water, in the end, we'll get a sugar solution. Here, the mass of both the ingredients and the final product will be the same.
Example:
Let us do an easy experiment to prove this law.
Step 1: Take one litre of water in a conical flask.
Step 2: Also, take \(5\) ml of salt solution in a test tube.
Step 3: Now, tie the test tube with a thread, leave it inside the conical flask and seal it.
Step 4: Mix the test tube solution with the water in the conical flask.
Step 5: Now, remove the thread and the test tube after the complete transfer of liquids.
Step 6: Let us now weigh the weight of the conical flask.
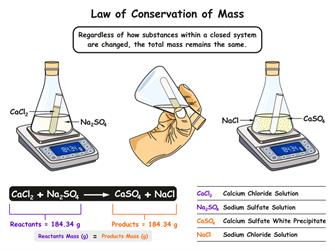
Observation: The mass of the reactants is equal to that of the products formed.
Result: The law of conservation of mass is being proved with this experiment. As the starting weight of the reactant is equal to \(1.00500\) litres, which is similar to that of the final products after the reaction.
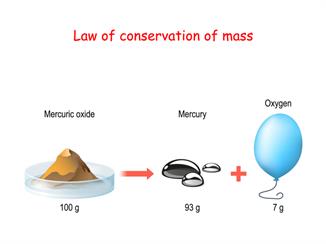
Similarly, we can also prove this by reacting other chemicals such as sodium chloride with copper sulphate, sodium sulphate, lead nitrate and so on.
Law of conservation of mass