
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoPure substances are those that are completely made up of one form of particle. Therefore, physical processes cannot distinguish pure substances from other forms of matter.
Example:
Salt, sugar, oxygen, copper, iron etc.,
Based on the kind of atom, we classify pure substances as
- Elements
- Compounds
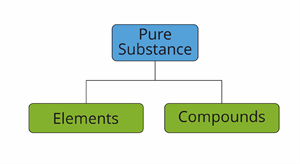
Classification pure substances
Compounds:
It is a form of matter created by combining two or more elements in a specific mass ratio. To decompose it into its constituent components, we use chemical methods.
Example:
Water\(H_2O\), oxygen \(O_2\), nitrogen dioxide \(NO_2\), salt \(NaCl\), and so on.
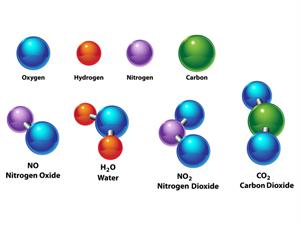
Elements:
Elements are the fundamental substances that cannot be separated into simpler substances by any chemical methods.
Example:
All the elements in the periodic table is an example for elements - such as Oxygen, Iron, etc.
In \(1661\), Robert Boyle became the first scientist to use the term 'element'.
Robert Boyle
A French chemist named Antoine Laurent Lavoisier \(1743-1794\) was the first to define an experimentally useful description of an element.

Antoine Laurent Lavoisier
According to Antoine Laurent Lavoisier, an element is a fundamental type of matter that cannot be broken down into simpler substances by chemical reactions.
Based on the physical and chemical properties, the elements are classified as
- Metals
- Non-metals
- Metalloids
Properties of Metals:
1. Malleable (can be drawn into thin sheets)

2. Ductile (can be drawn into wires)

3. Lustre (shine) with silvery grey or golden yellow

4. Sonorous (capable of producing a deep or ringing sound when hit)

Example:
Gold, copper, silver, iron, aluminium, etc.
Properties of Non-Metals:
1. Non-metals come in a wide range of colours.

2. They are bad conductors of heat and electricity and are known as insulators.

3. They lack lustre, sonority, and malleability since they are brittle and soft in nature.
Example:
Oxygen, nitrogen, sulphur, and diamond, etc.
Properties of Metalloids:
- Substances that show the characteristics of both metals and non-metals are known as metalloids.
- There are around \(8\) elements in the periodic table that are called metalloids.
Example:
Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), Polonium (Po).
Note:
- Mercury (metal) and bromine (non-metal) are two elements that exist as liquids at room temperature.
- Diamond is a non-metal which is hard and lustre in nature.


Pure Substance | Element or Compound | Reason |
| Water (\(H_2O\)) | Compound | Can be separated as Water = Hydrogen + Oxygen |
| Lead (\(Pb\)) | Element | Cannot be separated |
| Phosphorous (\(P_4\)) | Element | Cannot be separated |
| Sodium Chloride (\(NaCl\)) | Compound | Can be separated as Sodium Chloride = Sodium + Chloride |
| Sulphur (\(S_8\)) | Element | Cannot be separated |