PDF chapter test TRY NOW
Solution Properties:

A solution is a homogeneous mixture made up of the same proportions of any two or more substances.
2. Size of the particles.
2. Size of the particles.

It is not possible to see the particles of a solution through naked eyes because these particles are smaller than in diameter.
3. Scattering effect.
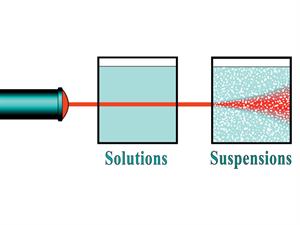
The solution is a well-mixed homogeneous mixture, and the solution particles' size is so tiny. So, we cannot see the light path when a beam of light passes in the solution. It is so because particles does not scatter light passing through the solution.
4. Stable solution.

In the filtration process, you cannot separate the solute particles from the mixtures because the solution is well mixed. As a result, the solute particles do not settle down even if it is left undisturbed.
The concentration of the solution:
Concentration is known as the ratio of solute in a solution to either solvent or total solution, and it refers to the amount of a substance per defined space.
Thus, the proportions of solute and solvent present in a solution determine the concentration of the solution.
a. Depending on the amount of solute in the solution, it may be called a dilute or concentrated solution.
1. Dilute solution: Dilute solution refers to a solution with a minimal volume of solute. This can also be known as an unsaturated solution.
2. Concentrated solution: A concentrated solution is a solution that contains a significant volume of solute.
b. Based on the dissolving limit of solvent and solute, the saturation can be classified into two types as a saturated solution and an unsaturated solution.
1. Saturated Solution: At a given temperature, a saturated solution is one in which no more solute can be dissolved.
2. Unsaturated solution: If the solution completely dissolves, leaving no substances remaining at the bottom is called an unsaturated solution.
Example:
Let us experiment to understand this concept clearly.
Step 1: Take some water in a glass and
Step 2: Add a small amount of sugar to that.
Step 1: Take some water in a glass and
Step 2: Add a small amount of sugar to that.
We can observe that the sugar is dissolved completely. Now, add more and observe. Still, it dissolves well. This kind of entirely dissolved solution is called an unsaturated solution.
Now, add more sugar and heat the solution slowly.
Similarly, if we add more coffee powder to water, it will stay at the bottom without dissolving.
Note: Remember that if the temperature is increased, then the dissolving power will also increase.
Observation:
But at a particular point, if you add more sugar, it stops dissolving and settles at the bottom of the glass, as the water attained its maximum dissolving limit known as saturation. Therefore, this kind of saturation is known as a saturated solution.
Similarly, if we add more coffee powder to water, it will stay at the bottom without dissolving.
