PDF chapter test TRY NOW
The preparation of common salt, drying wet clothes, meat, fish, hair in the sun, ironing of cloth are examples of evaporation. Evaporation is one of the basic purification methods used in our day to day life.
Evaporation method:
The process of evaporation begins with a solution. One compound is dissolved in another in a solution. For example, the separation of sugar solution and saltwater can be done with the help of this method. We are undoing reversing the dissolving process with evaporation.

Formation of salt by evaporation
Evaporation:
When the substance from the liquid state is boiled, it changes into a vapour. The change of state from liquid to vapour is known as evaporation. The process of evaporation occurs below the temperature of the boiling point of a substance.
- We are getting rid of the solvent so that the solute can stand on its own. If the solute is a gas, this will be difficult, but it will be relatively easy if the solute is a liquid or solid.
- During evaporation, the liquid's temperature falls and to maintain the temperature; they absorb the heat from the surroundings.
- The evaporation process speeds up when the surface area is large compared to the smaller surface area. This process occurs at the upper surface of the liquid.
- The increase in temperature, wind speed and decreased level of humidity speeds up the process of evaporation.
Example:
Let us now check the evaporation with small activity by separating the coloured component with the blue or black ink.
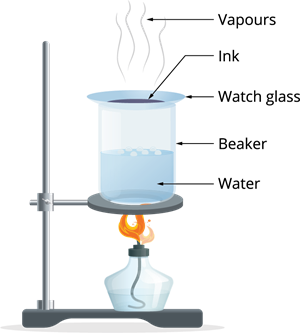
Evaporation apparatus
Step 2: After some time, the water in the beaker starts boiling. The heat is transferred to the watch glass, and evaporation takes place.
Step 3: Continue the process of heating until no changes seen on the watch glass.
Observation: The ink which was kept in the liquid form gets evaporated, leaving over the coloured component in the watch glass, which show the ink is a mixture of water with dye.
Result: Using the evaporation process, we can distinguish the volatile portion (solvent) from its non-volatile solute.
Factors affecting evaporation:

- Impurities present in liquid makes the process slower.
- The liquid evaporates faster when the temperature is hot; if not, the process will occur at a slower rate.
- If the humidity is higher, then evaporation is slower.
- The wind speed also determines the rate of evaporation.
