
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoSimple distillation method is used to extract a liquid from a solution i.e separating the solvent that dissolves substances from a solute - the substance that had dissolved.
Distillation:
Principle: The objective of the distillation process is to obtain the pure form of liquid from the solution.
Distillation is the combination process of evaporation and condensation.
Evaporation + Condensation = Distillation
Evaporation + Condensation = Distillation
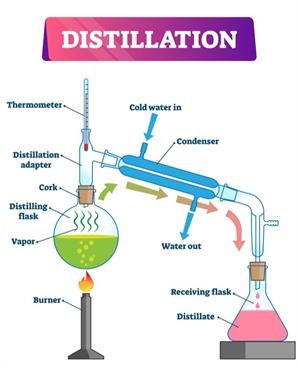
Simple distillation apparatus
Simple distillation works well when the substances to be separated have entirely different boiling points, such as salt and water. Water (Boiling point \(100\ °C\)) and propanone (boiling point \(60\ °C\)), but it is an overly simplistic process for separating a mixture of liquids, particularly when the boiling points of the constituents are near.
Function:
- The solution is heated to vaporise the liquid. The water is used to cool the liquid's hot steam; then, it is condensed into pure liquid.
- Initially, we heat the solution to vaporise the liquid. To cool it down the hot stream of the liquid, we use water. Then, it condensed into pure liquid.
Example:
Let us see how we can separate the solution of acetone and water.
Step 1: Fill a distillation flask halfway with the mixture.
Step 2: Set up the apparatus as show in the figure.
Step 3: Slowly heat the mixture, keeping an eye on the thermometer.
Step 4: Acetone evaporates, condenses in the condenser, and is deposited at the condenser outlet.

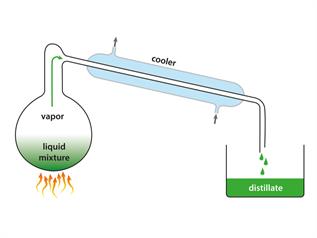
Simple distillation process
Observation: In the distillation flask, water is left behind.
Distillation condenser
Result: The boiling point of acetone is \(56°C\), and water is \(100°C\). Therefore, acetone evaporates faster compared to water when heated. Thus, finally, the mixture of acetone and water is separated with the help of simple distillation.
Application:
- There is a piece of evidence that humans were using the distillation process since \(3000\) BC (approximately). This process is used to make a distilled water.
- Few countries use the distillation process to convert seawater into drinking water.
- This process also used to refine the crude oil and to purify the alcohol, and more.
Advantages of simple distillation over fractional distillation:
- This process is faster compared to fractional distillation.
- It requires less energy input.
- The apparatus is simpler and cost-effective.
Simple distillation