
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoSeparating funnel:
Separatory funnels, also known as separation funnels, are popular in chemistry laboratories. Immiscible liquids are separated from their solutes using these funnels.
The funnel is normally pear-shaped, made of glass, and comes with a stopper and a stopcock.
Separating funnel principle:
According to the theorists, immiscible liquids separate into layers based on their densities.
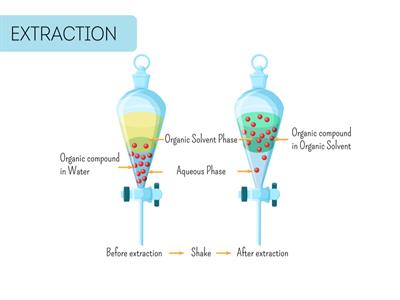
Separating funnel
Immiscible liquids are the substances that would not blend together to form a single phase. Immiscible liquids, such as oil and water, float on top of each other.

Immiscible liquids
In the funnel, the layers can divide based on relative densities, with the lowest density liquid rising above the higher densities.
Example:
Separation of two immiscible liquids: Let us see, if we can separate kerosene oil from water.
Step 1: In a separating funnel, pour the kerosene oil and water mixture.
Step 2: Allow it to sit undisturbed for a while to form separate layers of oil and water.
Step 3: Open the separating funnel's stopcock and carefully drain out the lower layer of water.
Step 4: As soon as the oil enters the separating funnel's stopcock, close it.

Separating funnel
Observation: We can find two different layers of liquids, the heterogeneousliquids.
Result: Separation of these immiscible liquids is possible with the help of a separating funnel.
Applications:
- Immiscible liquids are being separated from their solutes.
- This method of extracting iron from its ore removes the lighter slag from the top of the furnace, leaving the molten iron at the bottom.