PDF chapter test TRY NOW
We heard about a few physical properties of matter in the previous chapter.
Physical properties can be observed and defined, such as colour, structure, size, hardness, rigidity, fluidity, density, melting point, boiling point, etc.
Physical changes:
It is a process in which the substance experiences change in its physical properties such as, shape, size, colour, volume, appearance, state, etc. No new substances are formed, and they are reversible in nature.
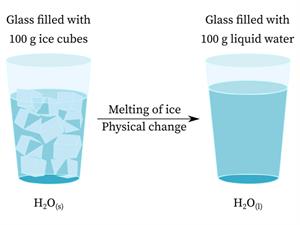
Physical changes
While ice, water, and water vapour have distinct appearances and physical properties, they are chemically identical.

Oil and water
Water and cooking oil are both liquids, but their chemical properties vary.

Oil catches fire

Water used to put off the fire
They have distinct odours and are more flammable. Oil burns in the air while water extinguishes fuel. It is oil's chemical property that separates it from water.
Chemical changes:
These are nothing but the change in the chemical properties of matter, resulting in the creation of new substances. A chemical reaction is another word for a chemical change.

Paper turns into ash (chemical change)
Burning is a chemical transformation. During this step, one material interacts with another, resulting in a chemical shift.
