
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIn our day to day life, we come across many liquid state matters, such as the water we drink, the oil we use for cooking, the fruit juice we often drink, etc. These are all the matters in a liquid state.
We can observe that the liquids do not have a certain shape, but it can take the shape of the container when we pour it in the container.
For example, we might have seen the juice bottles. The liquid inside the bottle takes the shape of the bottle.

Liquid characteristics
Liquid State:
- A intermediate phase between the solid and gas.
- Liquids have a fixed volume but no fixed shape.
- And it has a capability to take the shape of the container without changing it's volume.
Intermolecular Force on Liquid:
Take a look at the below figure. You can notice that the molecules inside the liquid are comparatively free than the molecules in the solid.
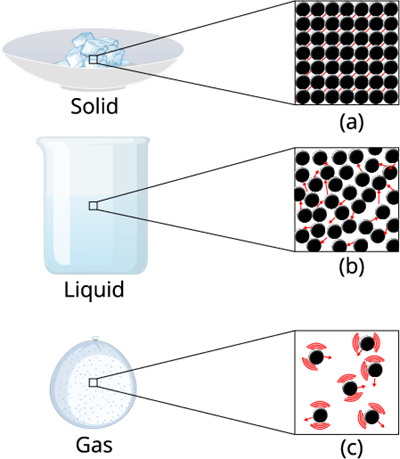
Intermolecular forces matters
Therefore it is evident that in liquid-state, particles move freely and have greater space between each other as compared to particles in the solid-state.
The intermolecular force in liquid is not high as compared to solid, but it's higher than gas.