PDF chapter test TRY NOW
While your mother cooks in the kitchen, you can sense the delicious aroma coming to you even though you're in another room. Have you ever wondered how that smell came to you?

Gas characteristics
And, we all like balloons, right? But, have you ever wondered how the balloon seller fill those a large number of balloons in a short span of time?
Have you ever wondered, what would be the science behind the LPG that we use in our home for cooking purpose or the oxygen cylinder that we use to treat the patients in the hospitals?
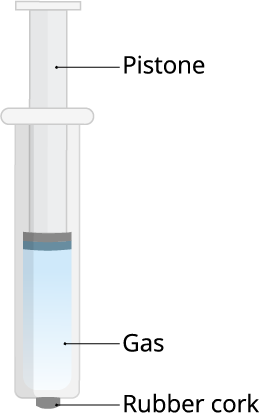
And, the answer to all the above questions is gaseous particles.
Gas Particles:
Gases are highly compressible as compared to solids and liquids. In the gaseous state, the particles move about randomly at high speed.
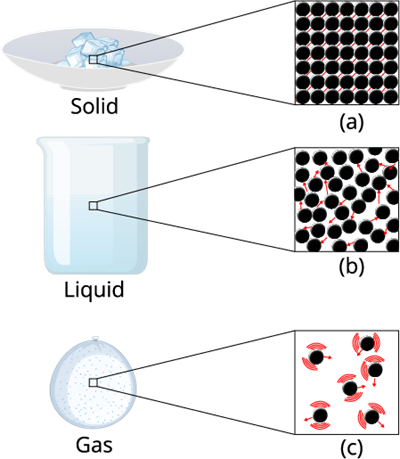
Intermolecular forces matters
Because of this property, we can use the gases in LPG in highly compressed condition. If we compress the gas particles, we can store it in a small container too.
We know that gas particles have more freely movable particles, so it can travel through the air easily. That's why the aroma of a food reaches to you even though you're in a separate room.
Intermolecular Force:
The inter molecule force in the gaseous substance is weaker as compare to the liquid and solid. This weaker inter molecule force makes the gaseous matter to move freely in the air.
