
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWe can distinguish the characteristics of particles of matter into three types.
1. Particles of matter have space between them.
2. Particles in a matter are continuously moving.
3. Particles of matter attract each other.
1. Particles of matter have space between them.
In previous exercise, we experimented with water and sugar/salt. And, we learnt that when we dissolve salt in water, the particles of salt/sugar get into the spaces between particles of water.
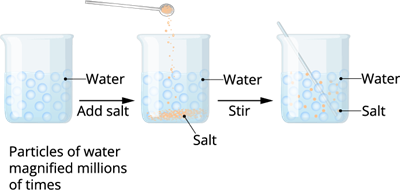
This experiment clearly shows that there is enough space between the particles of matter.
Spacing particles
Similarly, when we make lemonade (lemon juice), tea or coffee, we can observe the same principle.
2. Particles in the matter are continuously moving.
Particles of matter moves continuously, but slowly. To clarify this concept, let us do one experiment.
Step 1: Take a glasse or bowl filled with water.
Step 2: Drop of blue ink slowly along the sides of the bowl.
Leave it undisturbed for a few minutes. And, note down what you see after a couple of minutes.
You could see that the ink is spreading to water slowly. This proves that the particles in a matter are moving.

Moving particles
In another case, if we increase the temperature, the motion of the particles will also increase; therefore, their kinetic energy also increases.
Therefore, we can say that with increase in temperature, the kinetic energy of the particles also increases.
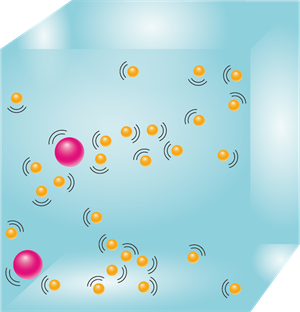
3. Particles of matter attract each other.
The particles of matter attract each other due to the various forces acting between them. These forces keep the particles together. The strength of the forces of attraction varies from one kind of matter to another.

Attracting particles