
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoTransportation of substance across the plasma membrane is an essential function. In general transportation of substance can be classified into two types.
1. Active transport
2. Passive transport
1. Active transport
2. Passive transport
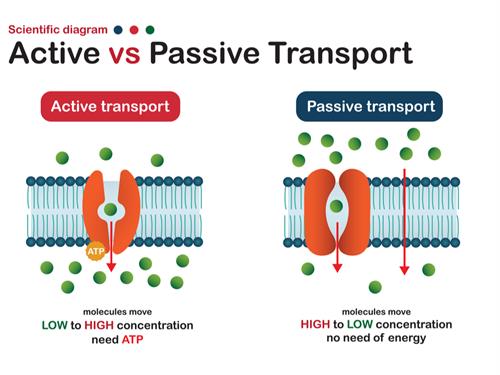
Active transport:
Active transport involves the movement of ions or molecules from regions of lower concentration to those of higher concentration. As this process is against the direction of natural process, it happens only with the expense of energy. Here energy is utilised in the form of \(ATP\) molecules (Adenosine triphosphate).
Note: In a natural process, materials will move from higher concentration region to lower concentration region.
Passive transport:
Passive transport involves the movement of ions or molecules without any expense of energy; i.e., it is a naturally driven process. This types of transportation are happening across the plasma membrane.
This transport is of two types.
1. Diffusion
2. Osmosis
Diffusion
Diffusion is a naturally driven process by which substances (solid, liquid & gases) move from a higher concentration to a lower concentration region.
Example:
If we spray a perfume bottle at one end of the room, its fragrance spreads across the room through the process of diffusion.
Diffusion of Gases in cells:
In cells, diffusion occurs in solids, liquid and gases. Diffusion helps in gaseous exchange.
Example:
1. Carbon dioxide, a cellular waste product, leaves the cell via the plasma membrane because its concentration is higher inside the cell and lower outside.
2. Similarly, since there is a higher concentration of oxygen outside the cell and a lower concentration inside the cell, oxygen enters the cell via the plasma membrane.
2. Similarly, since there is a higher concentration of oxygen outside the cell and a lower concentration inside the cell, oxygen enters the cell via the plasma membrane.
Diffusion also helps in getting nutritive substances from the environment.
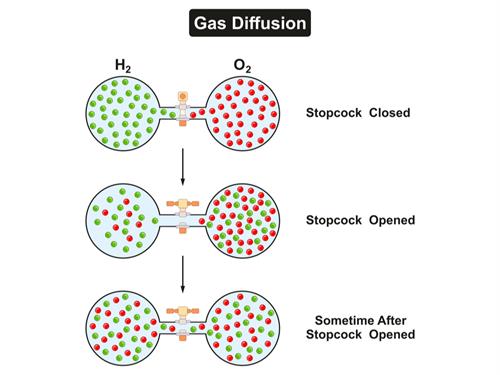
In the above picture, Hydrogen and Oxygen are present in two separate bulbs connected by a valve. When the valve between them is opened, both the gases tend to mix (second picture). As time passes (third picture), hydrogen and oxygen are in equal proportions in both the bulbs. This mixing is due to diffusion. Hydrogen and oxygen tend to move from high concentration areas towards other regions of comparatively lower concentration, resulting in an equal mix of both the gases across all regions. (State of equilibrium).
Diffusion of Liquid in cells:
Water movement inside and outside the cells happens through diffusion. This diffusion process in water is called as osmosis.
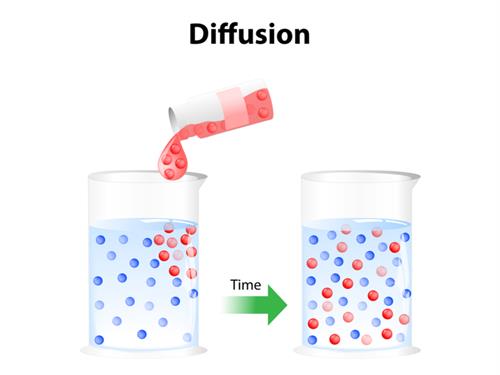
In the above picture liquid in the beaker (shown as blue coloured molecules) is added with another liquid (shown as red coloured molecules). After some time, two liquids tend to mix each other and reaches an equilibrium state, where both the liquids are in equal proportions throughout the beaker.
Important!
Diffusion is faster in gases than solids and liquids.