PDF chapter test TRY NOW
Osmosis in a cell is the movement of water from a region of higher water concentration to the region of low water concentration through a selectively permeable membrane till the equilibrium is reached. This kind of movement is along the concentration gradient. There is no expenditure of energy involved in this process. Osmosis is a form of passive transport.
For example, absorption of water through plant roots is happening by the process osmosis.
For example, absorption of water through plant roots is happening by the process osmosis.
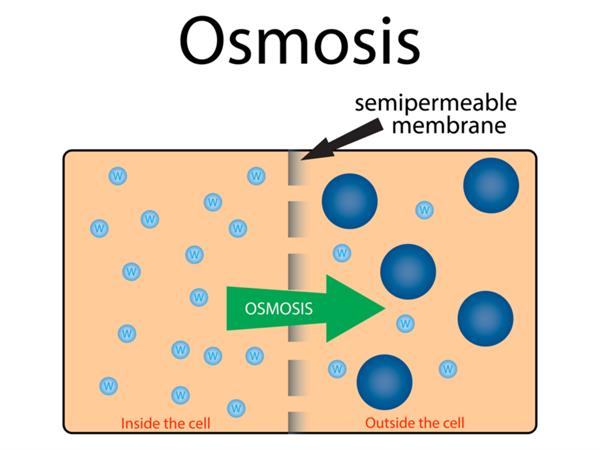
Above picture shows the inside and outside of a cell and the process of osmosis through the plasma membrane. You can see the water concentration inside the cell is high ( shown as small blue colour molecules) compared to outside of the cell, which induces water movement from inside of the cell to outside of the cell through the semi-permeable membrane until the equilibrium. This occurrence is called as osmosis.
Note:
- Solution is a mixture of a solute dissolved into a solvent in a homogenous manner.
Example: Sugar solution is a homogeneous mixture of sugar (solute) dissolved in water (solvent). - If a solution or medium is termed as highly concentrated, then the solute concentration in it is higher.
- If a solution or medium is termed as highly dilute or low concentrated then solute concentration is lower.
- If the solution or medium is termed as highly water concentrated then the concentration of water is high in it.
For example, water moves across a selectively permeable membrane from a low concentrated region to a highly concentrated region through osmosis. It can also be stated as water moves from a high water concentrated region to a low water concentrated region through osmosis.
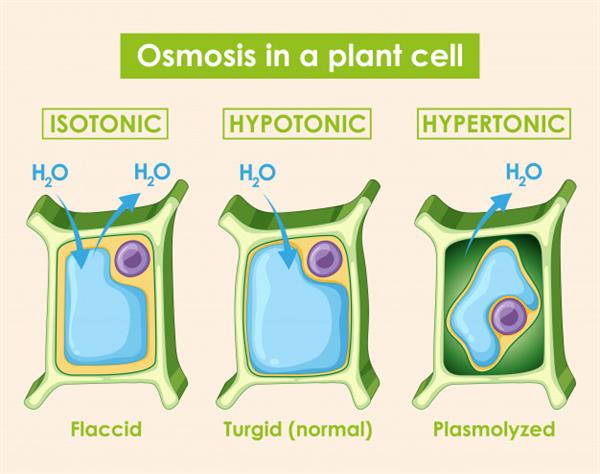
There are three types of osmotic solutions which can present across the plasma membrane.
1. Hypotonic solution
2. Hypertonic solution
3. Isotonic solution
2. Hypertonic solution
3. Isotonic solution
Hypotonic solution:
If the solution surrounding the cell has higher water concentration compared to inside of the cell (or outside solution is very diluted ), then the cell gains water by osmosis. Such a solution is termed as Hypotonic solution.
Water is free to cross the cell membrane in both directions. But the amount of water moving into the cell is higher than the amount of water moving out. So, the overall movement of water will be towards the inside of the cell. As a result, cell swells up (Endosmosis).
Water is free to cross the cell membrane in both directions. But the amount of water moving into the cell is higher than the amount of water moving out. So, the overall movement of water will be towards the inside of the cell. As a result, cell swells up (Endosmosis).
Hypertonic solution:
Suppose the solution surrounding the cell has a lower concentration of water than inside of the cell (i.e. outside solution is very concentrated). In that case, the cell will lose water by osmosis. Such a solution is termed as Hypertonic solution.
Water is free to cross the cell membrane in both directions, but this time more water leaves from the cell than enters it. As a result, the cell gets shrinked (Exosmosis).
Isotonic solution:
Water is free to cross the cell membrane in both directions, but this time more water leaves from the cell than enters it. As a result, the cell gets shrinked (Exosmosis).
Isotonic solution:
It is the solution that has the same osmotic concentration as inside the cell, and there is no movement of water molecules between the membrane. Such a kind of solution is known as Isotonic solution.
Water is free to cross the cell membrane in both directions, but the amount of water moving in remains the same as the amount of water moving out. So, there is no overall movement of water. As a result, no overall change is observed, and the cell size remains the same.
Water is free to cross the cell membrane in both directions, but the amount of water moving in remains the same as the amount of water moving out. So, there is no overall movement of water. As a result, no overall change is observed, and the cell size remains the same.
Types of Osmosis
Endosmosis:
In a highly dilute environment (Hypotonic solution), water moves from the environment into the cell through osmosis. This process is called Endosmosis.
When we put a raisin inside a bowl of water, the raisin starts to swells up after a few days. This swelling up happens because the raisin is surrounded by a hypotonic solution which causes the water movement into the raisin from the solution.
In a highly dilute environment (Hypotonic solution), water moves from the environment into the cell through osmosis. This process is called Endosmosis.
When we put a raisin inside a bowl of water, the raisin starts to swells up after a few days. This swelling up happens because the raisin is surrounded by a hypotonic solution which causes the water movement into the raisin from the solution.
Raisin (Dry grapes):

Dry grape
Exosmosis:
In a highly concentrated environment (hypertonic solution), watermoves from inside a cell to its environment through osmosis. This process is called exosmosis.
Suppose we put grapes in a highly concentrated sugar solution, i.e., in hypertonic solution, water molecules move from the grapes to the sugar solution. This process is called as exosmosis. It results in shrinking of grapes.
Difference between osmosis and diffusion
S.No | Osmosis | Diffusion |
1 | It is the movement of water from the region of higher concentration to a region of lower concentration through a semi-permeable membrane. | It is the movement of substances from the region of higher concentration to a region of lower concentration. |
2 | It requires a semi-permeable membrane. | It does not require any semi-permeable membranes |
Reference:
https://www.freepik.com/free-vector/diagram-showing-osmosis-plant-cell_5982972.htm
