
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWhen a neutron strikes a uranium nucleus (\(U^{235}\)), the process of fission occurs, creating three neutrons. These three neutrons produce a fission reaction in three more uranium nuclei in the sample, thus resulting in nine neutrons. These nine neutrons undergo fission and make another twenty-seven neutrons, and so on. This type of reaction is known as a Chain reaction.
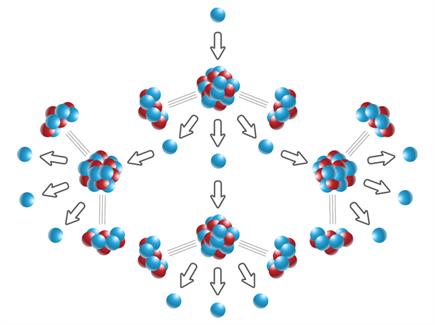
Chain reaction
A chain reaction is a self-propagating process in which the number of neutrons multiplies rapidly in a geometrical progression.
Generally, two types of chain reactions are possible. They are
(i) Controlled chain reaction
(ii) Uncontrolled chain reaction
(i) Controlled chain reaction
(ii) Uncontrolled chain reaction
Controlled chain reaction:
The number of neutrons released in a controlled chain reaction is always one. This is accomplished by using a neutron absorber to absorb the excess neutrons released during the process, leaving only one neutron to produce further fission. As a result, the reaction is kept under control.
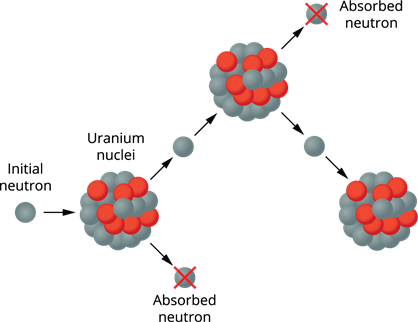
Controlled chain reaction
The energy released by a regulated chain reaction can be used for many constructive purposes. In a nuclear reactor, the controlled chain reaction is employed to produce energy in a predictable manner over a long period.
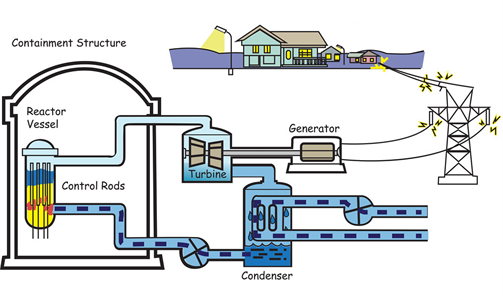
Energy generation from a nuclear reactor
Uncontrolled chain reaction:
The quantity of neutrons multiplies endlessly in an uncontrolled chain reaction, causing fission in a huge mass of fissile material. This causes a massive amount of energy to be released in a fraction of a second. This chain reaction is employed in an atom bomb to create an explosion.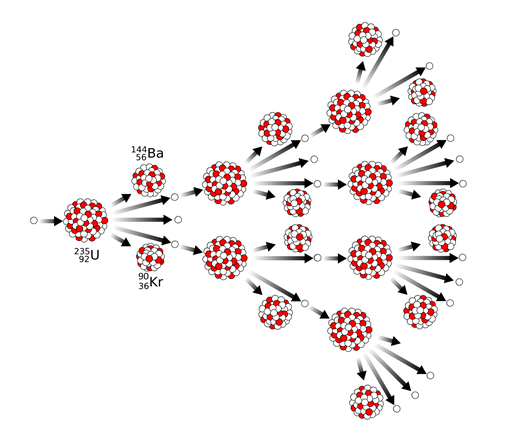
Uncontrolled chain reaction
It is calculated that \(10^{23}\) uranium atoms undergo fission in \(one\ minute\) in an uncontrolled chain reaction.
The main difference between the controlled and uncontrolled chain reactions is that an enormous amount of energy is released in a short span of time and causes an explosion in an uncontrolled reaction. Whereas in the controlled reaction, the energy is released in a slow and controlled manner, which can be effectively used without causing any destructive explosions.
Reference:
https://live.staticflickr.com/5500/10713741876_eb52a0c66e_b.jpg